| |||||||||||||||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||||||||||||
|
This process goes on all the time, but so far away that it's hard to even observe, much less model with accuracy or replicate in a laboratory. Despite the obstacles, however, astronomers, physicists, and chemists are building an increasingly detailed picture of the complex processes going on in these regions known as interstellar clouds. If chemistry is the science of molecular transformations, it's fair to say that chemistry begins in these cold, dark regions between the stars. As older stars end their lives, whether they explode or just gently break apart, the matter they once contained spews out into the interstellar medium, where it collects under the influence of gravity to form clouds. Some of this matter is in the form of very small dust particles, typically about one-tenth of a micrometer in size. Spectroscopic evidence reveals these tiny, smokelike particles to be of two types: Some are metallic silicates; others are carbonaceous. But most of the matter that blows out of stars is atomic in nature. By mass, about 99% of the cloud is made up of atoms in the gas phase and 1% is dust particles. Hydrogen is by far the most abundant element found in these clouds, with carbon, oxygen, and nitrogen each also present at concentrations about four orders of magnitude lower than that of hydrogen.
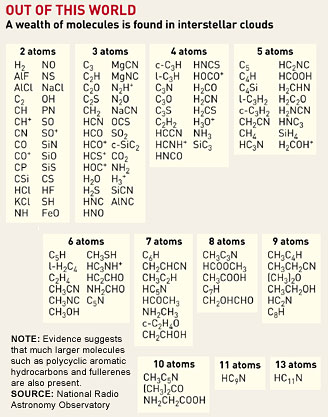
The key feature of the chemistry going on in these clouds is that it takes a long time. The densest parts of the clouds have something like 10,000 molecules per cubic centimeter--a better vacuum than any that can be obtained in a laboratory, notes Eric Herbst, professor of physics and astronomy at Ohio State University. That makes collisions in the gas phase very rare. Herbst explains, "If you are a gas atom, you might collide with another one every day or so," instead of the billions of collisions per second that take place in a gas at atmospheric pressure. Nevertheless, over the billions of years that the clouds exist, collisions occur and molecules form. Spectroscopic evidence confirms the presence of a wide variety of molecules both in the gas phase and in the form of icy mantles that coat the surfaces of the dust grains. In fact, almost every atom in these clouds is bound up into some sort of molecule. More than 120 kinds, ranging in complexity from diatomics to molecules containing 13 atoms, have been identified in the gas phase of these clouds. About half of these are organic molecules, including representatives of many of the familiar classes of terrestrial organics, such as nitriles, aldehydes, alcohols, acids, ethers, ketones, amines, and amides, as well as long-chain hydrocarbons. In addition, infrared spectral features strongly suggest that much larger polycyclic aromatic hydrocarbons containing 100 or more carbon atoms are also present in the clouds. "It's the same molecules, but it's a different kind of chemistry than you get on Earth." SPECTROSCOPIC DATA on the gas phase of interstellar clouds is more detailed and less ambiguous than that of the ices. Information about the gas-phase molecules comes mostly from rotational spectroscopy done in the microwave region, which astronomers refer to as radioastronomy. It can be done from the ground. The ices, on the other hand, can be studied only with IR spectroscopy, which is hard to do from the ground because of the interference of water vapor in Earth's atmosphere. The best data come from satellite-based instruments, particularly from a satellite of the European Space Agency called the Infrared Space Observatory or ISO. Data from this satellite, which operated from late 1995 until May 1998, continue to be analyzed and to add to the basic information chemists and astronomers have about these clouds. Thirty years ago, Herbst, then a graduate student at Harvard University, and his research adviser, physical chemist William Klemperer, proposed that much of the gas-phase chemistry in interstellar clouds would be based on ion-molecule reactions. Cosmic radiation could produce energetic ions to initiate these reactions, which, because they require little or no activation energy, could take place even at temperatures as low as 10 K. The model provides an explanation for the unusual mix, by terrestrial standards, of molecules seen in interstellar clouds, and it still provides the core of current understanding of the gas-phase chemistry of the clouds. Although gas-phase chemistry is important, reactions on the surfaces of particles as well as in the ice layers that form around them "are definitely going on," Herbst says. The most abundant molecule in interstellar space, molecular hydrogen, wouldn't be the most abundant if it were made only in gas-phase reactions. Two colliding hydrogen atoms carry so much energy that there has to be a third body--like a dust particle--to absorb some of the energy, or the nascent molecule will tear itself apart. It seems likely, Herbst says, that the mix of molecules formed in surface chemical reactions is different from the products of gas-phase chemistry. "The molecules that form through surface chemistry are more hydrogenated, more saturated in the chemical sense, than the molecules formed in the gas phase," he says. One of the distinctive features of gas-phase interstellar molecules is that they are highly unsaturated, with many multiple bonds, particularly between carbon atoms. BUT THE CHEMISTRIES mix. "Some of the ices can evaporate into the gas phase, and, of course, the gas-phase molecules can condense into the solid phase," Herbst says. "If one looks in the warmish regions, where stars are forming, the mix of molecules in the gas phase becomes much more saturated. The interpretation is that the ice mantles have melted, and all the hydrogen-rich molecules that formed in the ice are now in the gas phase." Herbst notes that understanding in any detail the surface and solid-state chemistry going on in interstellar clouds is a tough problem. The dust particles build up mantles of ices composed mostly of frozen water, but containing, as well, carbon dioxide, carbon monoxide, and smaller amounts of other molecules like formaldehyde and methanol. The ices are not uniform either in their chemical composition or, probably, in their physical structure. "When you don't know exactly what the surface is, it doesn't make life any easier," Herbst says. "These things are very dependent on the exact nature of the material, and in our models we have to make a lot of simplifying assumptions." For example, he explains, "we assume that most of the chemistry occurs via diffusion on the surface. In other words, if you imagine two species landing on a surface and being weakly bound, they diffuse until they find one another. That's the picture, and there's laboratory evidence for it. But it's probably not the whole picture. There are other possibilities, such as species being bound strongly, not moving at all, and being attacked by another species from the gas phase." Although ion-molecule reactions are very important in the gas-phase chemistry occurring in interstellar clouds, they are not the only class of chemical reactions that may be going on there. A growing body of laboratory experiments demonstrate that interactions between neutral atoms or molecules can also occur at the very low temperatures found in interstellar clouds. These reactions very likely add complexity to the gas-phase chemistry of the clouds. For the past decade, physical chemists Ian W. M. Smith of the University of Birmingham, in England, and Bertrand Rowe of the University of Rennes, in France, have been using a technique first developed to study ion-molecule reactions at extremely low temperatures to study similar reactions between neutral molecules. Some of these reactions, such as reactions of CN radicals with unsaturated hydrocarbons to form cyanopolyynes, occur fast enough at 10 K that they need to be included in theoretical models of cloud chemistry, Smith and Birmingham colleague Ian R. Sims have suggested.
"That speed is sort of helpful, because we can only measure fast reactions with the technique that we have," Smith notes. "We don't go chasing the rate constants of reactions we have good cause for thinking won't be fast." The technique that Smith and Rowe use is known as CRESU, a French acronym for reaction kinetics in uniform supersonic flow. Reactant molecules and a carrier gas are cooled to very low temperatures by expansion through a specially designed nozzle. The molecules drop to temperatures as low as 7 K under conditions that are uniform enough to study reaction rates. Pulsed lasers are used to generate free radicals and to observe the rate at which they are removed. Although the temperatures in these experiments are comparable to those of interstellar clouds, the densities are not. "But we believe in the law of mass action," Smith says, "so we believe that it's possible to scale down to the densities that are in an interstellar cloud." The technique is relatively straightforward, he points out, if at least one of the reactants is readily available as a pure gas. Experiments become much more complicated if both reagents are rare or unstable, so that they have to be synthesized just prior to conducting an experiment. Unfortunately, that's the case with many of the species of interest in interstellar clouds. "We are now reaching the difficult point," Smith says, "because we have used up almost all the reactions that are relatively easy to study with our technique. Of course, we hope that by making measurements on the relatively simple reactions that we have looked at, theorists will be able to test their theories. Then they may be able to predict rates of reactions that we may never be abxle to measure, or at least not in the foreseeable future." As the experiments have become more difficult, Smith has become more selective about which ones he undertakes, trying to choose those that are likely to provide information that has significance for astrochemistry. ONE CURRENT EXPERIMENT looks at the reaction between oxygen atoms and hydroxyl radicals. "There's a bit of a mystery why there's not more dioxygen in interstellar clouds than has been measured," Smith explains. One suggestion is that the reaction he's now studying, which models predict to be a prime source of O2 molecules in the clouds, might actually be slower than the rate inferred from data at higher temperatures.
Other researchers are attempting to replicate the chemistry taking place on the dust grains in interstellar clouds. Initial efforts have focused on the formation of molecular hydrogen, the simplest molecule occurring in interstellar clouds, but also, arguably, the most important one. Hydrogen is the most abundant molecule in the universe, notes Valerio Pirronello, professor of physics at the University of Catania, in Italy. That fact alone makes it important to understand how it forms. In interstellar clouds, where it's five orders of magnitude more abundant than any other molecule, it also shapes the rest of the chemistry taking place, at least in the gas phase. Theoreticians have proposed since the early 1960s that almost all molecular hydrogen in interstellar clouds forms on the surface of dust particles. In 1997, Pirronello, along with physics professors Gianfranco Vidali of Syracuse University and Ofer Biham of Hebrew University, in Jerusalem, succeeded in performing this synthesis in the laboratory under conditions that closely resemble those found in interstellar clouds. In their ultra-high-vacuum experiment, a beam of hydrogen atoms, cooled to 150 to 200 K, was directed at a very cold (5 to 15 K) olivine surface, a silicate mineral chosen for its resemblance to silicate interstellar dust particles. As predicted by theory, some of the atoms stuck to the surface and diffused across it until they encountered other hydrogen atoms. When two atoms reacted, a fraction of the excess energy was absorbed by the silicate, stabilizing the newly formed molecule, which then desorbed from the surface, to be detected by mass spectrometry. The researchers have also performed this experiment on two other low-temperature surfaces: amorphous carbon and frozen water. "We've covered the three classes of surfaces that are believed to be present in the interstellar medium," Pirronello says. "The biggest difference between the process that occurs in space and the one we can simulate in the laboratory is that we are using surfaces, rather than single grains," Pirronello notes. That difference is likely to change some of the details of the process, he suggests. For example, energy absorbed from the reaction might raise the temperature of a small grain, although this doesn't happen on a macroscopic surface. Pirronello and his colleagues have also studied how carbon dioxide might form in interstellar clouds. Carbon dioxide is an unusual interstellar molecule because it is observed only in the ices that form under suitable conditions around dust grains; it's not present in the gas phase. That makes it "a very good probe of surface reactions on grains," Pirronello notes. In their experiments, the researchers deposit carbon monoxide and oxygen atoms on a substrate at interstellar temperatures. They then cover these reactants with a layer of water ice and slowly heat the system, eventually reaching temperatures of 140 to 150 K. They find that CO and oxygen atoms move through the water-ice layer to combine and form carbon dioxide. The newly formed carbon dioxide stays in the ice until the ice sublimes, when the researchers detect CO2 in the gas phase. Pirronello and Vidali's CO2 experiment is an important one, says astrochemist Steven B. Charnley of the National Aeronautics & Space Administration's Ames Research Center and the SETI Institute, both in California. "Prior to this experiment, people had assumed that, to form any carbon dioxide in the ices, you had to have some kind of energetic processing by ultraviolet light or high-energy particles," he explains.
Williams' own interest is star formation. But, as he explains, "that means I'm interested in understanding what an interstellar cloud is like. Part of that cloud has to collapse and the density has to go up by 20 orders of magnitude to make a star. That's not easy!" Gravity drives the process, but many other factors, including magnetism and temperature, are important, too. To understand the role of magnetism in star-forming clouds, Williams adds, he needs to know what ions are present. "But to understand that, you need to understand the whole chemistry of the cloud, because the chemistry is driven in part, at least, by ion-molecule interactions. And the molecules themselves are freezing out onto the grains to form ices. The fact that some molecules may stick to the grains and some don't affects the chemistry; it affects the level of ionization, and that affects the amount of magnetic support there is to the gas. All sorts of complicated things are happening." Williams and his colleagues are conducting experiments similar to the ones of Pirronello and his colleagues. The London project has two parts, theory and experiment. While one group shoots a beam of hydrogen atoms at about 100 K at a graphite surface that's kept at 10 K, another uses detailed quantum mechanics to calculate how the resulting hydrogen molecules should be distributed among various vibrational and rotational excited states. "The point of the experiment is to pick out precisely the molecules that are being made on the surface in particular vibration/rotation states," Williams explains. "We can work out their kinetic energy, too, using a time-of-flight mass spectrometer." There's not a perfect match yet between the calculated energy distribution and what's found in the experiments, Williams says. "We feel that there's some vibrational relaxation occurring on the surface as the molecule comes off, though we haven't confirmed that yet," he explains. "That's telling us, perhaps, more about how the molecule comes off the surface than we had understood before." The researchers are beginning to set up experiments to look at other chemical reactions--such as the formation of hydrides--that take place on the particles. Detailed theoretical calculations for hydride formation are also getting under way. "We think it's important to do the theory alongside the experiments," Williams explains. "The two feed off each other, and you get much more insight." Elsewhere, Helen J. Fraser, an astrochemist and postdoctoral researcher at the University of Leiden, in the Netherlands, is bringing the tools of surface science to the study of chemistry taking place on grain surfaces in interstellar clouds. "I focus, in particular, on what kind of heterogeneous chemistry could be going on," Fraser says. "Things that you see regularly in surface catalysis, like how molecules are coming to and from a surface and how they diffuse across a surface, are key to how this chemistry might be occurring." Fraser studies the ice that builds up on dust particles in the coldest parts of the clouds. In the lab, as in space, these are very complicated surfaces, she says. For instance, ices formed in lab experiments at the temperatures of interstellar clouds usually take on an amorphous structure, she says, but if they undergo any kind of processing, there's likely to be an irreversible phase change to a crystalline form. In space, similar processing could well be occurring when, for example, photons or cosmic rays strike the ice surface. "There's chemistry that can go on when molecules are frozen out next to each other and later diffuse and react, and there's chemistry that can go on when molecules from the gas phase go onto the surface and react directly," Fraser points out. "Both of those chemistries could be different, depending on the ice structures. So it's quite a big, messy problem that we have to solve." If chemistry is the science of molecular transformations, it's fair to say that chemistry begins in the cold, dark regions between the stars. TECHNIQUES DEVELOPED in the past decade primarily to study catalysis at surfaces are now being applied to interstellar chemistry, Fraser notes. Among the most important are facilities to conduct ultra-high-vacuum, low-temperature experiments that begin to approximate conditions in interstellar clouds. Even the best ultra-high-vacuum systems available in labs today, however, are still three orders of magnitude higher in pressure than conditions in the densest parts of interstellar clouds. Astrochemists are also making use of many of the noninvasive analytical techniques used in surface science, such as reflection/adsorption IR spectroscopy and low-energy electron diffraction. Fraser and physical chemist Martin R. S. McCoustra of the University of Nottingham, in England, have been studying ices that form from water, carbon monoxide, and mixtures of the two. "We understand quite a lot about water ice and carbon monoxide ice," Fraser says, "but when we put them together, desorption properties change and alter. We find that the spectroscopy can change quite significantly." In a recent series of experiments, Fraser and her Leiden colleagues have reexamined the formation of carbon dioxide in ices in the presence of an energy source such as ultraviolet light. Experiments conducted at Leiden and elsewhere beginning in the 1970s showed that carbon dioxide can form when an ice containing carbon monoxide is irradiated with UV light in the presence of oxygen atoms. In Fraser's experiments, as in the earlier ones, the ice is first exposed to UV radiation; then the radiation is switched off and the ice slowly heated. People had previously thought they saw CO2 formation during this thermal warming, Fraser says, but the new experiments show that this is not the case. By using isotopic substitution in all the reagents and altering the concentrations of the reagents over a much wider range than had been done in the earlier work, Fraser and her colleagues find that changes in the infrared spectrum for carbon monoxide seen in earlier experiments are the result of changes in the ice matrix around these molecules, rather than conversion of CO to CO2. The experiments also show that, CO2 forms more efficiently in the presence of UV radiation by reaction of carbon monoxide with hydroxyl radical rather than oxygen atoms, Fraser says.
These experiments get at fundamental questions, she says. "What happens if I bump a radical or an atom very gently onto an ice surface? Can I directly, even at 10 K, force a chemical reaction to occur, or do I have to take the surface to a higher temperature, like 50 or 60 K? Or do I have to embed these radicals and atoms into the ice, and later on, as the ice warms up, as it does in the environment around a baby star, is that when the complex chemistry occurs?" For theoreticians like NASA's Charnley, experiments like these verify what reactions are possible and help shape the pathways they use to predict the chemistry that may be taking place in interstellar clouds. "A big thrust of the past three or four years has been the influx of people with expertise in surface chemistry and surface physics who have started looking at these problems," Charnley says. THE MAJOR COMPONENTS of interstellar ices--water, methanol, formaldehyde, and formic acid--"seem to suggest that there's some kind of catalytic process going on there that involves atom addition reactions," Charnley says. Hydrogen seems to add to atoms or molecules already in the ice to form water, ammonia, methane, and probably also formaldehyde and methanol. It's likely, though, that formation of many of the more complex molecules found in interstellar clouds involves a combination of reactions taking place in the ices and in the gas phase, Charnley says. Models suggest that molecules like dimethyl ether, diethyl ether, methyl formate, and acetone don't form on the surface of grains, he says. Rather, they form in the gas from products of grain surface reactions. Spectral data can determine only the major components of interstellar ices because infrared spectroscopy can't detect anything that makes up less than about 1% of the ice. Gas-phase detection by radioastronomy is about 1,000 times more sensitive, so astrochemists look in the warmer regions of the clouds, where the ice mantles have evaporated, to try to piece together the chemistry that takes place in the ices. The trick, Charnley explains, is to "eliminate those species from the inventory that you know probably formed in the gas. By effectively partitioning the molecular composition of these warmer regions between species that truly formed on the grains and were evaporated from those that formed from these evaporated products, you can constrain the reaction pathways that we think could be happening on the surface of the grains." Charnley continues, "Of course, what you want to do as a theorist is to determine what the basic mechanisms are for forming molecules in grains. Once you understand that, then you can crank the handle, theoretically, and see which molecules should be coming out and at what abundances. Additionally, that's going to lead to the prediction of new molecules that should be detectable. The theoretical part is passed on to observers as a kind of finder's chart for new molecules to go and look for." It's already clear that the composition of interstellar clouds "isn't something you would pick out of a basic organic chemistry textbook," Charnley says. For example, the chemistry seems to favor formation of fairly long, linear species. Many of the small organic molecules common on Earth have not been observed, such as most three-carbon organic molecules that also contain oxygen. "It's the same molecules, but it's a different kind of chemistry than you get on Earth," Charnley says. "Most terrestrial chemistry is liquid phase, and there's none of that here. You have to look at the theoretical models, and not an organic chemistry textbook, to find the molecules we should be looking for in space." | |||||||||||||||||||||||||||||||||||||||
|
Chemical & Engineering
News | |||||||||||||||||||||||||||||||||||||||