Extensive quantity describing the progress of a chemical reaction equal to the number of chemical transformations, as indicated by the reaction equation on a molecular scale, divided by the
Avogadro constant (it is essentially the
amount of chemical transformations). The change in the extent of reaction is given by
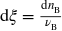
, where
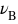
is the
stoichiometric number of any reaction entity
B
(reactant or product) and

is the corresponding amount.
Source:
PAC, 1996, 68, 149
(A glossary of terms used in chemical kinetics, including reaction dynamics (IUPAC Recommendations 1996))
on page 165
PAC, 1996, 68, 957
(Glossary of terms in quantities and units in Clinical Chemistry (IUPAC-IFCC Recommendations 1996))
on page 973
PAC, 1992, 64, 1569
(Quantities and units for metabolic processes as a function of time (IUPAC Recommendations 1992))
on page 1572
PAC, 1993, 65, 2291
(Nomenclature of kinetic methods of analysis (IUPAC Recommendations 1993))
on page 2295
Cite as:
IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8.
doi:10.1351/goldbook.



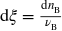 , where
, where 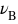 is the stoichiometric number of any reaction entity B
(reactant or product) and
is the stoichiometric number of any reaction entity B
(reactant or product) and
 is the corresponding amount.
is the corresponding amount.