- Home
- About Us
- Our Branches
- Special interest branches
- Camelid Branch
- Club Practitioners Branch
- Companion Animal Society
- Deer Branch
- Epidemiology and Animal Health Management Branch
- Food Safety, Animal Welfare and Biosecurity Branch
- Holistic Veterinary Society
- Industry Branch
- New Zealand Equine Veterinary Association
- Pig Veterinary Society
- Retired Veterinarians
- Society of Dairy Cattle Veterinarians
- Society of Sheep and Beef Cattle Veterinarians
- Veterinary Business Group
- Wildlife Society
- Regional branches
- Special interest branches
- Events & Online Learning
- Publications
- Vets & Vet Businesses
- The Public
- Newsroom
Just the cat's whiskers
By Keith McSporran, Gribbles Veterinary
Also known as vibrissae, whiskers are the visible extension of follicle–sinus complexes, present around the muzzle of many mammals: histological studies have even found remnants of them in our upper lips (Tamatsu et al, 2007). They are thought to have evolved with the eutherian mammals around 120 million years ago.
 Coat (pelage) hairs offer physical protection, insulation and rapidly adapting tactile sensitivity. Follicle–sinus complexes, on the other hand, are specialised sensory organs. As such, they are highly developed in nocturnal species and those that hunt or feed in low-visibility environments.
Coat (pelage) hairs offer physical protection, insulation and rapidly adapting tactile sensitivity. Follicle–sinus complexes, on the other hand, are specialised sensory organs. As such, they are highly developed in nocturnal species and those that hunt or feed in low-visibility environments.
Five sets are present in the cat, four of these on the head:
- the supra-orbitals above the eyes
- the genals – single or double units on the cheeks
- the mystacials, the most prominent, comprising a bilateral array of 12units in four rows on the whisker pad of the lateral muzzle
- the sub-mentals on the chin.
An additional group, the carpal–ulnar, is found on the caudo–medial foreleg near the carpus (Nilsson and Skoglund, 1965; Nilsson, 1969a; Nilsson, 1969b). Not all cats have whiskers; many Sphinx cats are devoid of both coat hairs and vibrissae.
 A rich folklore has developed around cats’ whiskers. Some see them simply as a means by which a cat can judge the width of a narrow space and so prevent itself getting stuck. This ignores the fact that their length appears to be genetically determined. The whisker pattern of a cat is thought to be unique, and once a cat reaches maturity, whisker length does not change with age or body condition. Others claim that whiskers allow cats to navigate in the dark.
A rich folklore has developed around cats’ whiskers. Some see them simply as a means by which a cat can judge the width of a narrow space and so prevent itself getting stuck. This ignores the fact that their length appears to be genetically determined. The whisker pattern of a cat is thought to be unique, and once a cat reaches maturity, whisker length does not change with age or body condition. Others claim that whiskers allow cats to navigate in the dark.
Rodents, such as the mouse and the rat, that “whisk” their whiskers can sense the surface contours of their immediate environment in the dark (Ritt et al, 2008). This is thought to be facilitated by the fact that the whiskers of rodents are graded in length and is probably mediated by intensity-encoded frequencies in the range 60–90Hz within the whisker (Gerdjiko et al, 2010).
Using just their vibrissae, rats can distinguish between aperture openings of 62 and 65mm (Krupa et al, 2001), between a smooth surface and one with grooves just 30µm deep (Carvell et al, 1990) and between sandpapers of different coarseness (Hipp et al, 2006). Such haptic (active touch) resolution and sensitivity is considered to be equal to that of the prehensile hand of primates. Cats, with their greater visual acuity and with whiskers of a similar length, do not show this behaviour and probably do not have this exquisite sensitivity. Despite this, the cat’s vibrissae allow it to sense air currents and subtle disturbances in its environment and so navigate and hunt in the dark. The more we learn about these organs, the more fantasy appears to merge with reality.
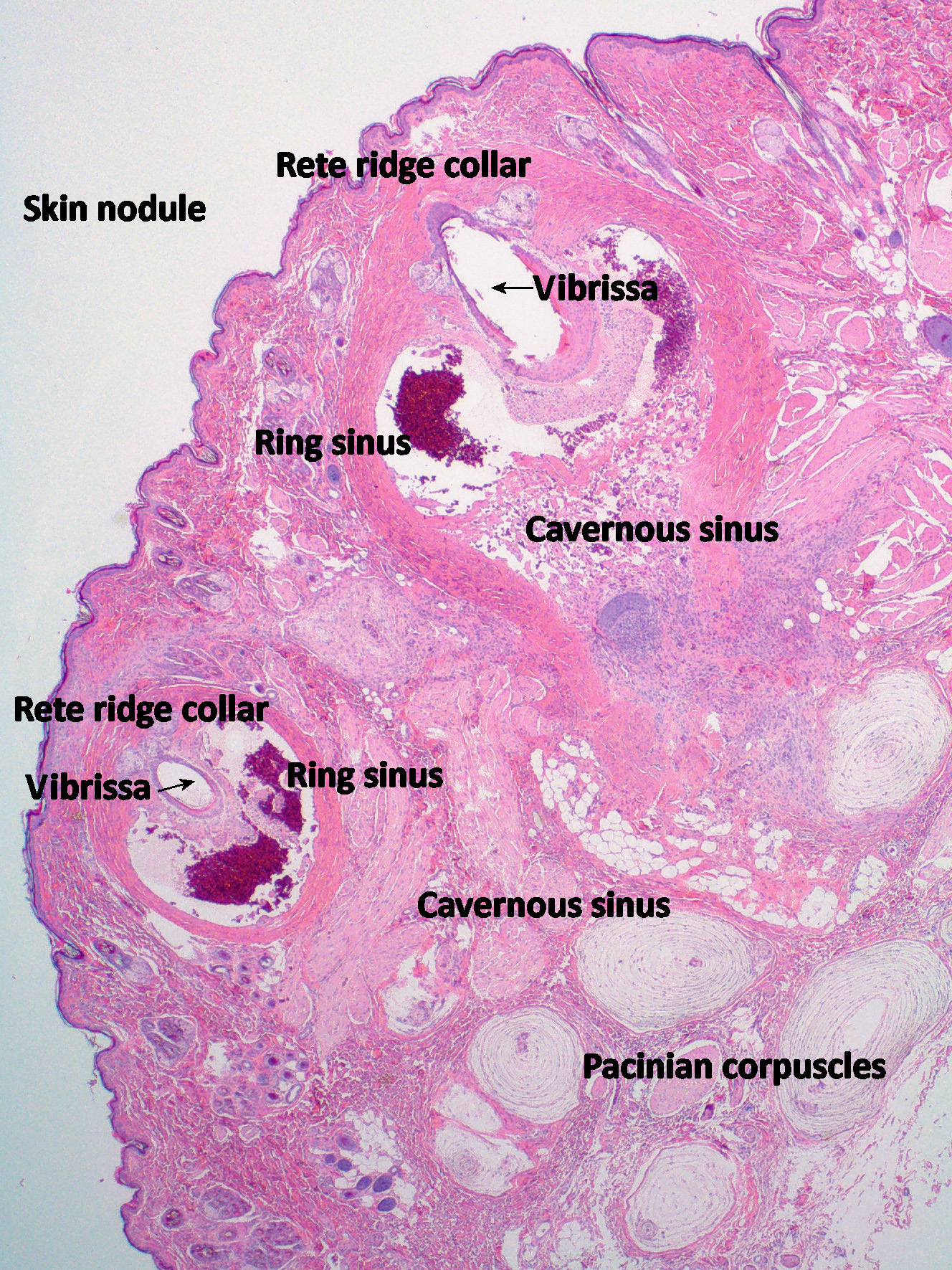
Follicle–sinus complexes are unique in that they have a vascular sinus surrounding the hair bulb, which is richly supplied by sensory receptors and nerves – hence the alternative name, sinus hairs. The hair shaft is also several times thicker than in pelage hairs, tapered in cross-section (pelage hair is of uniform diameter, except terminally) and extends up to three times further into the dermis. They are anchored in the skin by 3–5mm diameter, are easily palpable, nodular expansions of the epidermis and dermis, many recognisable by their dark red or black colour. At any one time, some may be devoid of hair shafts. This is because, like coat hairs, vibrissae also moult and it may be many weeks before they are replaced. Despite this, the appearance, bilateral symmetry and anatomical site of these units is usually enough to identify them.
Although the basic anatomy of follicle–sinus complexes is preserved across a range of mammalian species, considerable specialisation is seen both within and between species. Most are tapered and take the form of a truncated cone. Those of the pinnipeds such as the seal and the walrus, in which the mechano–receptor functions are best developed, have an oval, beaded profile, however. The follicle–sinus of these species is also surrounded by an additional vascular ring to maintain the nerves at body temperature in near freezing conditions, so critical are these sensors to their survival. Seals have 10 times more mystacial vibrissae than the cat, supplied by 320,000 myelinated axons (Marshall et al, 2006). By positioning their vibrissae perpendicularly to their direction of travel, seals can detect hydrodynamic trails left by fish, and use these to catch prey in zero visibility. Hydrodynamic trails remain for several minutes after water is disturbed by objects moving through it. Seals travelling at several metres per second can detect these in passing and follow them for up to 40 metres to catch prey. If voluntary movement of their vibrissae is restricted by attaching wire cages to their muzzles, however, they lose this ability. This explains why blind seals are able to feed themselves in the wild. The highly specialised vibrissae of the harbour seal and other phocids, with their oval cross-section and nodular profile, suppress vortex-induced vibrations, which would otherwise obscure the hydrodynamic trails of their prey.
 The follicle–sinus complexes of some species lose their hair shafts, often before or shortly after birth. These crypts become electro-sensors, such as those seen around the head of whales, dolphins and platypuses and are used to detect weak electric fields (Czech-damal et al, 2011). Yet others become pressure sensors and provide whales and pinnipeds with depth information while submerged.
The follicle–sinus complexes of some species lose their hair shafts, often before or shortly after birth. These crypts become electro-sensors, such as those seen around the head of whales, dolphins and platypuses and are used to detect weak electric fields (Czech-damal et al, 2011). Yet others become pressure sensors and provide whales and pinnipeds with depth information while submerged.
In species such as the cat, follicle–sinus complexes from the foreleg are anatomically different from those on the maxilla, and serve a different purpose. The mystacial vibrissae of the cat can be moved voluntarily in a rostral direction; the top two rows can even be moved independently of the lower two if necessary. The position of the mystacials indicates a cat’s state of mind: forward-facing mystacials signify an inquisitive and positively interactive mind-set, whereas backward-facing mystacials indicate a defensive or aggressive mood. During hunting, they are moved forward to envelop prey carried in the mouth and to provide information as to its disposition: cats deprived of mystacial vibrissae often have difficulty killing prey because they are unable to determine its orientation and are therefore less able to deliver a killing bite to the neck. Cats that have lost their sight adapt by moving the mystacial vibrissae forward permanently to assist them to negotiate their environment. Movement of the carpal vibrissae, on the other hand, is not under voluntary control: movement is controlled by involuntary muscles. Adrenergic stimulation increases the sensitivity of these fibres by making them more erect and more likely to come in contact with prey or obstacles. The hair disc, or rete ridge collar, of carpal hairs is positioned lateral to these vibrissae, enabling them to provide directional information, which the mystacial vibrissae do not, simply registering movement. They are most sensitive to movement away from the body, such as would occur if a mouse were to escape from a cat’s grasp. The special adaptation of these vibrissae is thought to be related to the nocturnal behaviour and poor visual accommodation of the cat, which has difficulty focusing on objects close to the body. Diurnal species such as the cheetah do not have carpal vibrissae.
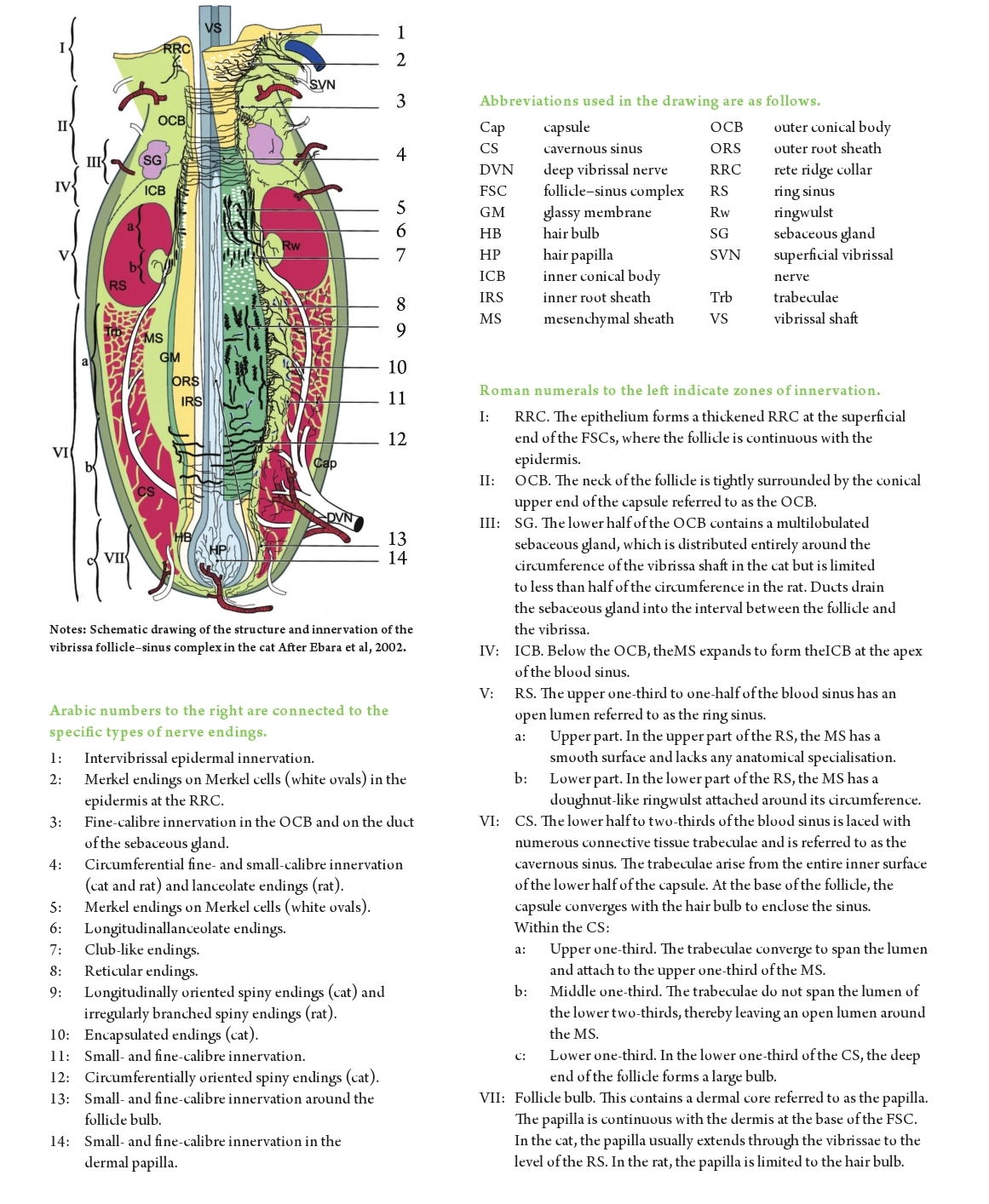
Early studies on the vibrissae of the cat indicated that there were as many as four different sensory receptors associated with these units. This was considered unusual (Nilsson, 1969b), one or two being far more common in most other species. However, recent histo-chemical studies have shown this to be a gross underestimate. That the follicles contain both slow-adapting and rapidlyadapting receptors has been known for some years. That these work in conjunction with the Merkel cell receptors in the rete ridge collar and the exquisitely sensitive vibration sensors, the pacinian corpuscles, in the adjacent dermis, alluded to an anatomically sophisticated sensory arrangement; however, it has now been shown that these complexes contain at least 13 different types of nerve endings (Figure 4 – after Ebara et al, 2002).
Such exquisitely engineered structures provide the cat with a wealth of information, which we are only now beginning to understand. In their own way, these organs are as complex as the eye, which they complement and sometimes substitute for, in the environments in which these species operate.
It is therefore regrettable when they are excised mistakenly as skin neoplasms – sometimes singly, but also as bilateral pairs of skin nodules.Cat owners may be concerned to find unexplained skin nodules on their pets. However, when these are situated on the face or the forelegs, we have cause to pause and consider the possibility that these may be “just the cat’s whiskers”. Some lumps are meant to be.
| References |
|
Carvell, GE and DJ Simons (1990) Biometric analyses of vibrissal tactile discrimination in the rat.J Neurosci 10(8):2638–2648. Czech-damal, NU, A Liebschner, GK Miersch, FD Hanke, C Marshall, G Denhardt and W Hanke (2011)Electroreception in the Guiana dolphin (Sotaliaguianensis)Proc Biol Sci Royal Soc, Jul 27. Ebara, S, K Kumamoto,T Matsuura, JE Mazurkiewicz and FL Rice (2002) Similarities and differences in the innervation of mystacial vibrissal follicle–sinus complexes in the rat and cat: A confocal microscopic study. J Comp Neurol 449(2): 103–119. Gerdjikov, TV, CG Bergner, MC Stuttgen, C Waiblinger andC Schwarz (2010) Discrimination of vibrotactile stimuli in the rat whisker system: Behavior and neurometrics. Neuron 65(4): 530–540. Hipp, J, E Arabzadeh, E Zorzin, J Conradt, C Kayser, ME Diamond andP Konig (2006) Texture signals in whisker vibrations. JNeurophysiol 95(3): 1792–99. Krupa, DJ, MS Matell, AJ Brisben, LM Oliveira andMAL Nicolelis (2001) Behavioral properties of the trigeminal somatosensory system in rats performing whisker-dependent tactile discriminations.J Neurosci21(15):5752–5763. Marshall, CD, H Amin, KM Kovacs andC Lydersen (2006) Microstructure and innervation ofthe mystacial vibrissal follicle–sinus complex in bearded seals, Erignathusbarbatus (Pinnipedia:Phocidae). Anat Rec ADiscov Mol Cell Evol Biol 288(1):13–25. Nilsson, BY (1969a) Structure and function of the tactile hair receptors on the cat’s foreleg. ActaPhysiolScand 77(4):396–416. Nilsson, BY (1969b) Hair discs and Pacinian corpuscles functionally associated with the carpal tactile hairs in the cat. ActaPhysiolScand 77(4):417–428. Nilsson, BY andCR Skoglund(1965) The tactile hairs on the cat’s foreleg. ActaPhysiol Scand 65(4):364–369. Ritt, JT, MT Andermann and CI Moore (2008) Embodied information processing: Vibrissa mechanics and texture features shape micromotions in actively sensing rats. Neuron 57(4): 599–613. Tamatsu, Y, K Tsukahara, H Mitsuyuki and S Kazuyuki (2007) Vestiges of vibrissal capsular muscles exist in the human upper lip. Clin Anat 20(6): 628–631. |
| Acknowledgements |
| I wish to thank Dr Satomi Ebara and his co-authors, John Wiley and Sons Ltd and The Journal of Comparative Neurology for permission to make use of a modified schematic diagram from the paper (Ebara et al, 2002) as Figure 4. The figure is copyright to John Wiley and Sons Ltd. Acknowledgement is also made for the use of the image of the cat’s whiskers (Figure 1) from the website todayIfoundout.com. Thanks also to Karen Cooper, Gribbles Veterinary Pathology Ltd. |