Survey of brain imaging techniques
with implications for nanomedicine
by
Stephen S. Flitman, MD
Cognitive Neurology Section, Barrow Neurological Institute,
Phoenix, Arizona, USA.
This is a draft paper for the
Eighth
Foresight Conference on Molecular Nanotechnology
Introduction
Nanotechnology has tremendous potential for advancing medicine. As a neurologist, I see incurable brain diseases daily: e.g., Alzheimer's and related disorders. However, diagnosis has improved greatly, driving progress as clinical trials of new treatments need cohorts of well-characterized patients. Non-invasive imaging of the patient's brain has proven invaluable in this context, evolving dramatically since the 1970s when it was realized that computer processing of X-ray densitometry could create tomograms (slices) of any X-ray permeable object such as the human skull. This led to now-ubiquitous CT (computed tomography) scans; prior to their advent, the only way to image the brain was via pneumoencephalography, which required introducing air into the ventricles of the brain for an air-contrasted skull X-ray, and early nuclear brain scans.
The 1980s brought the next level of achievement in the form of magnetic resonance imaging (MRI), which offers better resolution and discrimination than CT, which often fails to distinguish grey and white matter regions. MRI has excellent discrimination since instead of densitometry it relies on recording RF emissions stimulated from protons resonating in a high magnetic field by carefully timed pulse sequences. The theoretical maximum resolution of MRI is 100 microns, sufficient to see individual neurons in some parts of the brain, and the technique has been optimized in modern machines to the point where a whole brain volume can be acquired in under 3 seconds.
However, CT and MR show only static images of the brain's structure. SPECT and PET scans map brain function using a radioisotope adaptation of the CT principle; PET has superior resolution than single-photon SPECT because it uses positron-emitting isotopes and looks for the dual photons emitted by positron-electron annihilations. These scans detect regional blood flow rates which correlate to activity of large neuron populations in the cerebral cortex. Recently, a rapid MRI technique termed functional MRI has demonstrated better spatial and temporal resolution by imaging blood flow patterns at 200 ms per slice, opening the door to direct observation of cognitive activities such as willed movements, reading, hearing, or speaking.
A mature nanotechnology can be envisioned which grows out of the data provided by these techniques. Already there are CT- and MR-based systems which guide surgeons' instruments and help place neurostimulators to control Parkinson's disease and tremor. Non-invasive ablation of tumors by focused gamma rays is in use at a handful of centers, guided by MRI images. Given the existing ability to transform a composite structural-functional image to a standard coordinate system, it may be possible to devise motile nanodevices which take up addressable positions within the brain. Such devices could become visible on MRI by desequestration of paramagnetic gadolinium, communicating by varying their effective brightness on MRI, and respond like resonant protons to coded RF pulses. Critical applications of this technology would include delivering drugs and growth factors to specific locations, control of epileptic discharges, impression of sensory information directly into relevant cortex, which could open the way to a deeper understanding of how the brain functions in health and disease.
I. Early approaches at brain imaging
 Other than craniotomy, in which the skull is removed to observe the brain below, as is done routinely for neurosurgery, there existed until the age of X-ray (1) no other method to image the living brain. Conventional X-rays do not see the brain, however, due to its soft tissue density. The brain can be indirectly visualized on so-called plain films by filling the ventricles with air. This air-contrasted film is called a pneumoencephalogram. The procedure involved a spinal tap and injection of air into the spinal canal, which would naturally displace fluid and rise to the top (the patient's head) when the patient was turned upright. This was neither painless nor harmless, and went out of favor as soon as better techniques became available. In the adjoining figure, the skull dominates and the black curved portion in the middle is air outlining the ventricles Abnormalities such as tumor or hydrocephalus could be appreciated by skilled radiologists as a deviation of the appearance of the ventricles from normal. Large tumors of the pituitary gland could also be detected in this manner. Other than craniotomy, in which the skull is removed to observe the brain below, as is done routinely for neurosurgery, there existed until the age of X-ray (1) no other method to image the living brain. Conventional X-rays do not see the brain, however, due to its soft tissue density. The brain can be indirectly visualized on so-called plain films by filling the ventricles with air. This air-contrasted film is called a pneumoencephalogram. The procedure involved a spinal tap and injection of air into the spinal canal, which would naturally displace fluid and rise to the top (the patient's head) when the patient was turned upright. This was neither painless nor harmless, and went out of favor as soon as better techniques became available. In the adjoining figure, the skull dominates and the black curved portion in the middle is air outlining the ventricles Abnormalities such as tumor or hydrocephalus could be appreciated by skilled radiologists as a deviation of the appearance of the ventricles from normal. Large tumors of the pituitary gland could also be detected in this manner.
II. Static neuroimaging with computed tomography (CT)
 The work of Hounsfield (2) extended the usefulness of X-ray to examining solid objects (like the human body) in slices, termed tomography. The slice is a literal section through the body, and can be positioned axially (transversely, as in a top-down view), sagitally (longitudinally, as in a side view), or coronally (as in a view from front to back). CT scans were traditionally axial like the adjoining image. Using the filtered back-projection technique Hounsfield pioneered, these images are built from multiple overlapping radially projected X-rays. With more sensitive detectors and faster reconstruction times, modern CT delivers roughly the same radiation dose as a chest X-ray, and can be performed in a matter of minutes for acquiring a whole volume (a complete set of slices of the object of interest). Here we have a midtemporal slice from a normal subject, showing the ventricles in much greater detail, as well as the temporal lobes and the brainstem in the center. These are basically density plots, with air and water of lowest density and therefore black, and the bone of the skull and the metal of the headrest the highest density and therefore white. The brain is of intermediate density. The grey matter cannot be easily distinguished from the white matter but significant alterations, like bleeding or a large stroke, can be readily discerned to aid the neurologist. The work of Hounsfield (2) extended the usefulness of X-ray to examining solid objects (like the human body) in slices, termed tomography. The slice is a literal section through the body, and can be positioned axially (transversely, as in a top-down view), sagitally (longitudinally, as in a side view), or coronally (as in a view from front to back). CT scans were traditionally axial like the adjoining image. Using the filtered back-projection technique Hounsfield pioneered, these images are built from multiple overlapping radially projected X-rays. With more sensitive detectors and faster reconstruction times, modern CT delivers roughly the same radiation dose as a chest X-ray, and can be performed in a matter of minutes for acquiring a whole volume (a complete set of slices of the object of interest). Here we have a midtemporal slice from a normal subject, showing the ventricles in much greater detail, as well as the temporal lobes and the brainstem in the center. These are basically density plots, with air and water of lowest density and therefore black, and the bone of the skull and the metal of the headrest the highest density and therefore white. The brain is of intermediate density. The grey matter cannot be easily distinguished from the white matter but significant alterations, like bleeding or a large stroke, can be readily discerned to aid the neurologist.
III. Static neuroimaging with magnetic resonance imaging (MRI)
 Nuclear magnetic resonance (NMR) spectroscopy was first deployed in the 1960s to characterize molecules in solvents based on emitted radio frequency (RF) spectra when the solutions were placed in a strong magnetic field and perturbed with an incident RF pulse. NMR is heavily dependent on the paramagnetic coupling of atoms in the field, and this in turn depends on whether the atoms present are of particular isotopes. Hydrogen is strongly paramagnetic as its nucleus consists of a single proton, and since it is abundant in water, fat, and other tissues in the human brain, it is an excellent substrate for a brilliant extrapolation of NMR known as magnetic resonance imaging (MRI). The work of Lauterbur and Damadian (3,4) led a revolution in MRI that has culminated recently with the development of exquisitely sensitive diffusion-weighted imaging that can detect a stroke as it happens, magnetic resonance angiography (MRA) that rivals conventional angiograms for detail and ability to detect aneurysms, and high-speed "one-bang" techniques designed for imaging infants without need for sedation (5). As the field strength increases, the signal-to-noise ratio increases linearly: thus doubling the current standard 1.5 tesla MRI machine to 3.0 tesla only gains roughly double the spatial resolution; this is because that while the signal goes up as the square of the field strength, the noise goes up linearly, yielding only linear benefit (6). Nuclear magnetic resonance (NMR) spectroscopy was first deployed in the 1960s to characterize molecules in solvents based on emitted radio frequency (RF) spectra when the solutions were placed in a strong magnetic field and perturbed with an incident RF pulse. NMR is heavily dependent on the paramagnetic coupling of atoms in the field, and this in turn depends on whether the atoms present are of particular isotopes. Hydrogen is strongly paramagnetic as its nucleus consists of a single proton, and since it is abundant in water, fat, and other tissues in the human brain, it is an excellent substrate for a brilliant extrapolation of NMR known as magnetic resonance imaging (MRI). The work of Lauterbur and Damadian (3,4) led a revolution in MRI that has culminated recently with the development of exquisitely sensitive diffusion-weighted imaging that can detect a stroke as it happens, magnetic resonance angiography (MRA) that rivals conventional angiograms for detail and ability to detect aneurysms, and high-speed "one-bang" techniques designed for imaging infants without need for sedation (5). As the field strength increases, the signal-to-noise ratio increases linearly: thus doubling the current standard 1.5 tesla MRI machine to 3.0 tesla only gains roughly double the spatial resolution; this is because that while the signal goes up as the square of the field strength, the noise goes up linearly, yielding only linear benefit (6).
IV. Functional neuroimaging by autoradiography (SPECT, PET)
 CT and MRI enabled static imaging of the brain, a generally unchanging anatomic view which while accurately representing the physical appearance of the brain fails to assess how well the brain is working at the time of imaging. This critical distinction is termed functional imaging, since it is brain function rather than structure that is of interest. To be sure, non-imaging techniques like electroencephalography (EEG), magnetoencephalography (MEG), evoked potentials (ERP) and measuring various analytes in the cerebrospinal fluid also assess brain function, but are not constructing an image and so will not be further considered. (It is worth noting, however, that EEG has spawned a number of image-producing computer-assisted modalities, like quantitative or topographic EEG, which do share aspects of functional imaging with the techniques mentioned herein). CT and MRI enabled static imaging of the brain, a generally unchanging anatomic view which while accurately representing the physical appearance of the brain fails to assess how well the brain is working at the time of imaging. This critical distinction is termed functional imaging, since it is brain function rather than structure that is of interest. To be sure, non-imaging techniques like electroencephalography (EEG), magnetoencephalography (MEG), evoked potentials (ERP) and measuring various analytes in the cerebrospinal fluid also assess brain function, but are not constructing an image and so will not be further considered. (It is worth noting, however, that EEG has spawned a number of image-producing computer-assisted modalities, like quantitative or topographic EEG, which do share aspects of functional imaging with the techniques mentioned herein).
The first functional neuroimaging technologies were based on autoradiography, in which a radioactive tracer is injected into the subject and an apparatus such as a gamma camera or photomultiplier tube array detects emitted gamma rays as the tracer decays. The earliest "brain scans" were radionuclide-based and not tomographic, more like a fuzzy lateral brain image. Applying the Hounsfield principal to these early scans produced the above SPECT scan. SPECT stands for Single Photon Emission Computed Tomography, and underscores the fact that it is based on the decay of a single-photon emitting nuclide like Tc99m. The tracer is given intravenously and generally stays within the circulation, so that the image is essentially one of blood flow. Blood flows at different rates in the brain depending on location and on neural activity. This significant fact enables imaging techniques like SPECT to assess whether a particular task activates ("lights up") specific brain areas. It had been recognized since the mid-19th century that injuries to the left hemisphere of the brain preferentially damage language function, and that the left hemisphere controls muscles on the right side of the body (the right hemisphere controls the left side). These are examples of cortical specialization (or functional localization) and depart radically from earlier notions that the brain was a kind of "electric oatmeal", spatially bland and not well-organized. SPECT images can show which parts of the brain were active for the 20 minute period after injection; a rich literature exists which shows SPECT to crudely localize numerous brain functions. However, its spatial resolution is poor (around 1 cm at best) and it lacks temporal resolution due to the long half-life of the tracer.
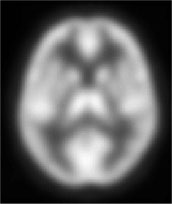 Positron emission tomography (PET) represents a significant refinement of the autoradiographic technique. PET scans rely on radioactive tracers that emit positrons, which include isotopes of carbon (11C), oxygen (15O), and fluorine (18F). Positrons cannot go far before encountering an electron and annihilating, producing a pair of gamma rays which shoot out in opposite directions. PET cameras are rings similar to CT scanners; a ring of photomultiplier tubes pick up the two gamma ray photons and can then use a technique similar to that used in CT to reconstruct slices of the brain (7). Such cameras are quite expensive because they require a fixed ring for each slice to be acquired concurrently. They are superior in terms of better spatial resolution (down to 5 mm in some cases) and temporal resolution (intravenous 15O-H2O blood flow scans can be acquired in under 30 seconds). The improved temporal resolution permits finer judgments to be made as to the area of brain activated by a particular task, and directly results from the higher sensitivity and shorter half-life of the tracers used. PET imaging has been a mainstay in cognitive neuroscience research, with substantial contributions from behavioral science and neuropsychology in terms of task selection and experimental design (8). Positron emission tomography (PET) represents a significant refinement of the autoradiographic technique. PET scans rely on radioactive tracers that emit positrons, which include isotopes of carbon (11C), oxygen (15O), and fluorine (18F). Positrons cannot go far before encountering an electron and annihilating, producing a pair of gamma rays which shoot out in opposite directions. PET cameras are rings similar to CT scanners; a ring of photomultiplier tubes pick up the two gamma ray photons and can then use a technique similar to that used in CT to reconstruct slices of the brain (7). Such cameras are quite expensive because they require a fixed ring for each slice to be acquired concurrently. They are superior in terms of better spatial resolution (down to 5 mm in some cases) and temporal resolution (intravenous 15O-H2O blood flow scans can be acquired in under 30 seconds). The improved temporal resolution permits finer judgments to be made as to the area of brain activated by a particular task, and directly results from the higher sensitivity and shorter half-life of the tracers used. PET imaging has been a mainstay in cognitive neuroscience research, with substantial contributions from behavioral science and neuropsychology in terms of task selection and experimental design (8).
V. Functional neuroimaging using magnetic resonance imaging (fMRI)
 It was recognized early in the MRI revolution by Rao and others that blood flow itself may be detectable by MRI, based on the paramagnetic effect of deoxygenated hemoglobin (9). This is true, with some caveats. MRI scanners equipped with rapid acquisition echo-planar hardware can acquire single slices in under 200 ms, which appear to be standard T2-weighted images (water is bright) with the addition that more rapidly flowing blood appears bright as well. These resultant T2*-weighted images are known as EPI (echo-planar imaging) and can be used to acquire whole brain volumes in 3 seconds or less, with resolution approaching 2 mm. The signal-to-noise ratio is not as good as PET, but the fMRI technique is superior in terms of enhanced spatial and temporal resolution and replicability: subjects can only be exposed to so much radiation per year, while fMRI has no injected contrast or tracer and does not appear to have any risks with repeat exposure. The adjoining image reveals activations in different brain regions occurring when a statistical contrast is performed between volumes acquired in two different tasks: pressing a button with the right index finger and pressing with the left index finger. While more than one area is activated, the principal focus of activation is in the motor strip on the right in this left vs. right comparison. Statistical contrasts are performed by essentially calculating an ANOVA across all of the images under consideration; this is the SPM approach pioneered by Friston and others (10). SPM was developed first for PET and SPECT and has been successfully migrated to the analysis of extended fMRI time series. It was recognized early in the MRI revolution by Rao and others that blood flow itself may be detectable by MRI, based on the paramagnetic effect of deoxygenated hemoglobin (9). This is true, with some caveats. MRI scanners equipped with rapid acquisition echo-planar hardware can acquire single slices in under 200 ms, which appear to be standard T2-weighted images (water is bright) with the addition that more rapidly flowing blood appears bright as well. These resultant T2*-weighted images are known as EPI (echo-planar imaging) and can be used to acquire whole brain volumes in 3 seconds or less, with resolution approaching 2 mm. The signal-to-noise ratio is not as good as PET, but the fMRI technique is superior in terms of enhanced spatial and temporal resolution and replicability: subjects can only be exposed to so much radiation per year, while fMRI has no injected contrast or tracer and does not appear to have any risks with repeat exposure. The adjoining image reveals activations in different brain regions occurring when a statistical contrast is performed between volumes acquired in two different tasks: pressing a button with the right index finger and pressing with the left index finger. While more than one area is activated, the principal focus of activation is in the motor strip on the right in this left vs. right comparison. Statistical contrasts are performed by essentially calculating an ANOVA across all of the images under consideration; this is the SPM approach pioneered by Friston and others (10). SPM was developed first for PET and SPECT and has been successfully migrated to the analysis of extended fMRI time series.
 Functional MRI lends itself to producing very impressive three-dimensional rendered brains. Here, the above sectional information is presented rendered onto a canonical brain using SPM software (Queens Square, London). The interpretation is still fraught with difficulty, however, because much inference needs to made about whether the subject was really doing the task, was he or she thinking about something else (and so activating other parts of the brain), did the scanner pick up real data or noise (much more noise is seen in fMRI than in PET, for example). It is typical to find papers which state categorically that a particular cytoarchitectonically defined Brodmann area is preferentially involved in a particular task, when the area mentioned is determined by comparing the coordinates of an activation to a standard atlas which by no means has been proven to reflect the borders of particular areas for all subjects. In fact, the converse is true; most Brodmann areas differ between individuals, and likely represent the result of a kind of evolution in utero, a conflict between neuronal cell populations for survival in the early infant brain, driven by adaptation to best serve senses like hearing and vision, genetics, and programmed cell death awaits those cells unlucky enough to be poorly connected or improperly located to serve the new brain adequately (11). While these imaging techniques have come a long way, understanding what is happening on the cellular level will not likely happen without the advent of a new technology, one which can reach down beyond the theoretical limits of MRI resolution at approximately 80 microns.(12) Functional MRI lends itself to producing very impressive three-dimensional rendered brains. Here, the above sectional information is presented rendered onto a canonical brain using SPM software (Queens Square, London). The interpretation is still fraught with difficulty, however, because much inference needs to made about whether the subject was really doing the task, was he or she thinking about something else (and so activating other parts of the brain), did the scanner pick up real data or noise (much more noise is seen in fMRI than in PET, for example). It is typical to find papers which state categorically that a particular cytoarchitectonically defined Brodmann area is preferentially involved in a particular task, when the area mentioned is determined by comparing the coordinates of an activation to a standard atlas which by no means has been proven to reflect the borders of particular areas for all subjects. In fact, the converse is true; most Brodmann areas differ between individuals, and likely represent the result of a kind of evolution in utero, a conflict between neuronal cell populations for survival in the early infant brain, driven by adaptation to best serve senses like hearing and vision, genetics, and programmed cell death awaits those cells unlucky enough to be poorly connected or improperly located to serve the new brain adequately (11). While these imaging techniques have come a long way, understanding what is happening on the cellular level will not likely happen without the advent of a new technology, one which can reach down beyond the theoretical limits of MRI resolution at approximately 80 microns.(12)
VI. MRI approaches to communicating with autonomous motile devices.
Progress in nanotechnology, once considered science fiction, has reached a point where it is not only reasonable but mandatory to begin to consider potential applications in medicine. The term nanomedicine, attributed to Freitas, implies a nanometer scale machine technology which will be used for scientific analysis of the human body, in vivo diagnosis of illness on a cellular or molecular level, and enhanced therapeutic capabilities, which may be envisioned to range from maintaining levels of important solutes in blood or cytoplasm, correcting genetic errors by directed modification of DNA, to large-scale reengineering of tissues, as might be considered in repair of trauma or burns(13). Neurologists have profited immensely from recent advances in genetics and molecular diagnosis, yet neurology as a whole lags far behind other fields in medicine because of the sheer complexity of the central nervous system. To go beyond what tools like MRI and fMRI have to offer, we need some means of analyzing the brain from within.
Autonomous motile devices, similar in concept to biological entities like bacteria, might serve this purpose. Injected into the bloodstream, they would disperse to defined locations to be deployed as sensors, reporting on local conditions, or as effectors, secreting a compound or eliminating an unwanted cell type as might comprise a brain tumor. The best approach would be to combine the two capabilities in a single unit, which hopefully could be produced in vast quantities cheaply (the essence of the nanotechnological revolution). The problem from an engineering perspective is how to guide them once in the labyrinth of the brain, as well as how to have them report to an outside observer what they have encountered.
MRI provides a natural means of determining macroscopic structure of the brain. It benefits from the intravenous injection of gadolinium, a paramagnetic contrast agent which outlines the blood vessels in a scan. Areas of inflammation, such as plaques in multiple sclerosis or viral infection, have more porous blood vessel walls which permits gadolinium to leak out, illuminating the lesion. The same is true of many tumors, to the extent that if the neuroradiologist sees a mass on MRI which enhances with gadolinium the diagnosis of brain tumor is virtually in hand. Could the paramagnetic enhancing effect of gadolinium be useful to our motile devices? It already is serving to report local conditions in terms of whether the blood-brain barrier is intact.
One way to make it useful would be to make it variable. Variable gadolinium, one may speculate, would be encapsulated so as to render its paramagnetic effect negligible. Unger et al. have reported on encapsulated forms of gadolinium, so chemically this is viable (14, 15). Then, on encountering a particular state locally, a motile device could decapsulate its stored gadolinium, which would be picked up by MRI as the subject possessing the devices lies on the scanner bed. Even if a more graded effect on the MRI perceived signal from that voxel were not achieved, a means of signalling can be obtained. If the effect were reversible, a binary code could be emitted by each device by virtue of whether its gadolinium was "on" or "off"; the time course of changes could be made quite fast with modern chemical techniques, and is necessarily limited by the temporal resolution of MRI (~50 ms for neurological applications). The clustering of signalling devices in a particular voxel would yield a higher signal intensity, and so convey information regarding the local concentration of a substance devices were programmed to recognize.
MRI also takes place in a magnetic field with superimposed gradients. These gradients are not consistently present, but shift in order to select slices and encode image data from a particular slice. It may be possible to design nanodevices which could sense gradients and use them in a manner analogous to a compass, orienting themselves along a particular axis. Directing them via coded pulses of EM radiation short enough for them to perceive may require ionizing radiation, which might be acceptable in low doses (X-ray, not gamma). It would be a more technical challenge to have them respond to RF pulses emitted by the MRI machine, perhaps by sensing resonant perturbations in their own constituent atoms.
Another approach would be to ask subjects to perform a particular task with known localization, such as button pressing, which would naturally increase blood flow in the activated region of the brain contralateral to the button pressed; now devices would be expected to preferentially follow the current and cluster in a region with higher blood flow. All of these designs can be tested using the reporting method described above.
Projected applications of this technique include realizing neurological aspects of much of what is contemplated for nanomedicine. Concrete examples include:
Selective destruction of brain tumor. Tumors of the brain are often gliomas, which have a tendency to grow between normal cells and are thus quite invasive and difficult to fully eradicate. Devices can be designed to recognize glioma cells and selectively destroy them, either by rendering their cell membranes permeable or by poisoning their protein synthesis machinery by selective release of an agent like diphtheria toxin. They can be guided to the tumor mass by exploiting the abnormal leaking blood vessels seen on MRI, and could perhaps be coaxed to take up positions corresponding to areas of abnormal enhancement on the patient's MRI.
Control of seizures in epilepsy. Epilepsy is defined as simply the tendency to have recurrent seizures, which are uncontrolled synchronized electrical discharges of many cells at once. The natural controls in the brain against seizures spreading and becoming manifest as a convulsion include inhibitory cells, special ion gates which clamp down on increased firing rates, and acidosis, or the tendency for active cells to lower their local pH, which is protective against seizures. Motile devices deployed to a known seizure focus, determined on clinical and electroencephalographic grounds, could detect seizures before they occur and damp down discharges by locally secreting inhibitors like diazepam or simply by lowering the local temperature via endothermic reaction. Macroscopic effectors like these have been shown to be effective in preventing initiation of seizures in animals by Robert Fisher, among others (16).
Sensory extension. Different sensory modalities are represented in specific brain areas. Blindness resulting from eye disease may be correctable by replacing the eye with a nanotechnological equivalent. Motile devices could take up retinotopic positions in visual cortex and once appropriately patterned, serve the same function as incoming neural projections from the visual radiation, now silenced by the loss of visual input. These devices could be stimulated transcranially by magnetic induction, or grow filaments out to form a contact point on the back of the head, there to connect to an external camera. It may be possible to use a similar approach to augment oneself with the capacity to "see" images as overlays over normal vision, such as information from the Internet or data stored in an implanted personal data accessory.
VII. Conclusions.
A number of modalities have been presented for brain imaging, ranging from early X-ray approaches, autoradiography, and magnetic resonance techniques. These have shown a general tendency towards increased spatial and temporal resolution with decreased exposure to ionizing radiation and increased dependence on advanced digital reconstruction hardware. Based on the physico-chemical data derived from the operational characteristics of MRI and injectable paramagnetic agents, a series of preliminary approaches to guidance and signalling from putative autonomous mobile devices is outlined, along with potential applications.
VIII. References
- Roentgen WC. Ueber eine neue Art von Strahlen (On a new kind of rays). Zur Geschichte der Physik an der Universitaet Wuerzburg 1894.
- Hounsfield GN. A method of an apparatus for examination of a body by radiation such as x-ray or gamma radiation. The Patent Office. UK, 1972.
- Lauterbur PC. Progress in n.m.r. zeugmatography imaging. Philos Trans R Soc Lond B Biol Sci 1980;289:483-7.
- Damadian R, Zaner K, Hor D, DiMaio T, Minkoff L, Goldsmith M. Nuclear magnetic resonance as a new tool in cancer research: human tumors by NMR. Ann N Y Acad Sci 1973;222:1048-76.
- Keller PJ. Fast(er) MR imaging. Neuroimaging Clin N Am 1999;9:243-52.
- Pipe J. Personal communication.:, 2000.
- Herscovitch P, Markham, J, Raichle ME. Brain blood flow measured with intravenous H215O. I. Theory and error analysis. J Nuclear Medicine 1983;24:782-789.
- Flitman S, O'Grady J, Cooper V, Grafman J. PET imaging of maze processing. Neuropsychologia 1997;35:409-420.
- Rao SM, Binder JR, Hammeke TA, et al. Somatotopic mapping of the human primary motor cortex with functional magnetic resonance imaging [see comments]. Neurology 1995;45:919-24.
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage 1998;7:30-40.
- Edelman GM. Neural Darwinism: selection and reentrant signaling in higher brain function. Neuron 1993;10:115-25.
- Van der Linden A, Verhoye M, Van Auderkerke J, et al. Non invasive in vivo anatomical studies of the oscine brain by high resolution MRI microscopy. J Neurosci Methods 1998;81:45-52.
- Freitas RA. Nanomedicine, Volume I: Basic Capabilities. Georgetown, Texas: Landes Bioscience, 1999.
- Unger E, Needleman P, Cullis P, Tilcock C. Gadolinium-DTPA liposomes as a potential MRI contrast agent. Work in progress. Invest Radiol 1988;23:928-32.
- Unger EC, MacDougall P, Cullis P, Tilcock C. Liposomal Gd-DTPA: effect of encapsulation on enhancement of hepatoma model by MRI. Magn Reson Imaging 1989;7:417-23.
- Fisher RS. Personal communication, 1999.
- Freitas RA. Personal communication, 2000.
|