| Ag | | |
| Ag | solute-volatilization interference
in flame spectroscopy |
| Ag+ | interfering substance
in electroanalytical chemistry |
| AgBr | | |
| AgBr | molecular rearrangement |
| AgCl | | |
| AgCl | gravimetric method |
| AgCl | gravimetric method |
| AgCl | gravimetric method |
| AgI | | |
| β-AgI | polymorphic transition |
| AgI | crystalline electrodes |
| α-AgI | polymorphic transition |
| AgNO3 | | |
| AgNO3 | gravimetric method |
| Ag2S | | |
| Ag2S | crystalline electrodes |
| Ag2S | crystalline electrodes |
| Al | | |
| Al | solute-volatilization interference
in flame spectroscopy |
| AlNb3 | | |
| Nb3Al | superconducting transition |
| Al2 | | |
| 2Al3+ | mean activity of an electrolyte in solution |
| Al2MgO4 | | |
| MgAl2O4 | solute-volatilization interference
in flame spectroscopy |
| Al3Cl | | |
| Al3Cl | ionization |
| Ar | | |
| Ar | gas-filled phototube |
| Ar+ | diamond by CVD |
| Ar+ | diamond-like carbon films |
| Ar | cryogenic sampling |
| Ar | Penning gas mixture |
| ArHOSe | | |
| ArSeOH | selenenic acids |
| As | | |
| As | poison in catalysis |
| As | photoconductive detector |
| AsH2 | | |
| H2As+ | arsanylium ions |
| AsH2O | | |
| H2As(=O)+ | arsanylium ions |
| AsH2O2 | | |
| HAs(OH)2 | arsonous acids |
| AsH3 | | |
| AsH3 | arsines |
| AsH3 | arsanes |
| AsH3O | | |
| H2AsOH | arsinous acids |
| H3As=O | arsine oxides |
| AsH3O2 | | |
| AsH3O2 | arsinic acids |
| AsH3O3 | | |
| AsH3O3 | arsonic acids |
| AsH4 | | |
| (H4As+) | onium compounds |
| AsH5 | | |
| AsH5 | arsoranes |
| As3H5 | | |
| H2AsAsHAsH2 | arsanes |
| As3H11 | | |
| H4AsAsH3AsH4 | arsanes |
| Au | | |
| Au | disproportionation |
| Au | disproportionation |
| Au | disproportionation |
| 197Au | nuclear quadrupole moment (spectroscopic) |
| AuFe | | |
| Au–Fe | spin-glass transition |
| BH | | |
| HB: | carbene analogues |
| HB+ | heteroconjugation |
| B2H | | |
| B–H–B | electron-deficient bond |
| B2H6 | | |
| B2H6 | electron-deficient bond |
| B5H9 | | |
| B5H9 | boranes |
| Ba | | |
| Ba2+ | mean activity of an electrolyte in solution |
| BaO3Ti | | |
| BaTiO3 | ferroelectric (antiferroelectric) transition |
| BiH3 | | |
| BiH3 | bismuthanes |
| BiH3 | bismuthines |
| BiH4 | | |
| (H4Bi+) | onium compounds |
| Br | | |
| Br | addition reaction |
| Br+ | halonium ions |
| Br | fragmentation |
| Br | molecular rearrangement |
| Br | telomerization |
| Br− | leaving group |
| Br | sulfonium compounds |
| Br | allylic substitution reaction |
| BrH | | |
| BrH | leaving group |
| BrH2 | | |
| (H2Br+) | onium compounds |
| C | | |
| 12C | relative micellar mass |
| C | repulsive potential-energy surface |
| 12C | molecular ion in mass spectrometry |
| C | transferases |
| C | dipolar compounds |
| C | potential-energy (reaction) surface |
| C | multiply labelled |
| –C= | heteroarenes |
| C | dipolar compounds |
| C | dipolar compounds |
| C | methylotrophic microorganisms |
| C | dipolar compounds |
| C | dipolar compounds |
| C | tautomerism |
| 13C | chemical shift,  in NMR in NMR |
| CB | | |
| BC | repulsive potential-energy surface |
| B–C | potential-energy (reaction) surface |
| CBrCl3 | | |
| CBrCl3 | telomerization |
| CBr4 | | |
| CBr4 | rotator phase transition |
| CCaO3 | | |
| CaCO3 | monotropic transition |
| CaCO3 | monotropic transition |
| CCl | | |
| C–Cl | coordination |
| CClF3 | | |
| CF3Cl | background concentration (level)
in atmospheric chemistry |
| CCl2 | | |
| :CCl2 | carbenes |
| CCl2F2 | | |
| CF2Cl2 | background concentration (level)
in atmospheric chemistry |
| CCl3 | | |
| Cl3C. | chain transfer |
| CCl3 | telomerization |
| Cl3C− | carbanion |
| Cl3C. | telomerization |
| CH | | |
| –CH= | dipyrrins |
| –CH= | quinones |
| C–H | σ, π (sigma, pi) |
| CH | agostic |
| CH | carbaboranes |
| C–H | steric isotope effect |
| C–H | symbiosis |
| C–H | persistent |
| HC… | carbynes |
| CHN | | |
| HN+≡C− | isocyanides |
| HC≡N | cyanides |
| HC≡N | isocyanides |
| C=NH | imines |
| H≡CN | nitriles |
| CHNO | | |
| HOC≡N | isocyanates |
| HC≡N+–O− | fulminates |
| HN=C=O | isocyanates |
| HOC≡N | cyanates |
| HON=C: | fulminates |
| CHNS | | |
| HSC≡N | thiocyanates |
| CHNSe | | |
| HSeCN | selenocyanates |
| CHO | | |
| –CHO | characteristic group in organic nomenclature |
| CHO2 | | |
| –COOH | characteristic group in organic nomenclature |
| CH2 | | |
| H2C: | alkylidenes |
| CH2 | tropyl radicals |
| H2C.+ | carbynium ions |
| CH2 | tropylium ions |
| CH2 | homoconjugation |
| –CH2– | pyrromethenes |
| H2C | silylene |
| H2C.+ | carbene radical cations |
| :CH2 | carbenes |
| –CH2– | meso structures in polymers |
| H2C: | carbenes |
| –CH2– | hydrocarbylene groups |
| CH2Cl | | |
| ClCH2– | organyl groups |
| CH2Cl2 | | |
| CH2Cl2 | α-addition (alpha-addition) |
| CH2N2 | | |
| CH2=N=N | isoelectronic |
| CH2=N2 | diazo compounds |
| HN=C=NH | carbodiimides |
| CH2Na2O4 | | |
| Na2CO3.10H2O | efflorescence |
| CH2O | | |
| CH2O | smog chamber in atmospheric chemistry |
| CHOH | oxo compounds |
| CH2O | fragmentation |
| CHOH | prochirality |
| CH3 | | |
| CH3 | allylic intermediates |
| CH3 | benzylic intermediates |
| .CH3 | radical (free radical) |
| CH3 | fragmentation |
| CH3 | σ, π (sigma, pi) |
| CH3 | dimerization |
| CH3 | colligation |
| CH3+ | even-electron ion |
| CH3 | abstraction |
| CH3 | dimerization |
| CH3 | fragmentation |
| CH3 | bond-dissociation energy,  |
| CH3 | insertion |
| CH3 | chain reaction |
| CH3 | chain reaction |
| CH3Cl | | |
| CH3Cl | substitution reaction |
| CH3Cl | molecular rearrangement |
| CH3ClO2S | | |
| CH3ClO2S | acyl halides |
| CH3F | | |
| CH3F | molecular laser |
| CH3I | | |
| CH3I | identity reaction |
| CH3IMg | | |
| MeMgI | organometallic compounds |
| CH3N | | |
| CH3N: | nitrenes |
| CH3N: | carbene analogues |
| CH3NO2 | | |
| CH3NO2 | azinic acids |
| CH3NO2 | carbamates |
| CH3NO2S | | |
| CH3N=S(=O)2 | sulfonylamines |
| CH3NaS | | |
| CH3S−Na+ | thiolates |
| CH3O | | |
| –CH2OH | uronic acids |
| OCH3 | π-electron acceptor/donor group |
| CH3O+ | oxylium ions |
| CH3S | | |
| CH3–S– | sulfenyl groups |
| CH3S. | sulfenyl radicals |
| MeS– | organyl groups |
| CH3S+ | sulfenylium ions |
| CH4 | | |
| CH4 | stoichiometry |
| CH4 | molecular entity |
| CH4 | isotope pattern in mass spectrometry |
| CH4 | reduced species |
| CH4 | spectator mechanism |
| CH4 | isotopologue |
| CH4 | composition of pure air in atmospheric chemistry |
| CH4 | multiply labelled |
| 13CH4+ | isotope pattern in mass spectrometry |
| CH4 | bond energy (mean bond energy) |
| 12CH4+ | isotope pattern in mass spectrometry |
| CH4 | bond energy (mean bond energy) |
| CH4 | bond-dissociation energy,  |
| CH4 | symbiosis |
| CH4 | abstraction |
| CH4N2O | | |
| CH4N2O | isoureas |
| CH4N4 | | |
| H2NN=CHN=NH | formazans |
| CH4O | | |
| CH4O | substitution reaction |
| CH4O | oxidative coupling |
| CH4O | line formula |
| CH3OH | Brønsted acid |
| CH4O | α-addition (alpha-addition) |
| (CH3OH2+) | lyonium ion |
| CH3OH | amphiprotic (solvent) |
| CH4O4 | | |
| C(OH)4 | ortho acids |
| CH4Si | | |
| H2Si=CH2 | heteroalkenes |
| CH5 | | |
| CH5+ | coordination number |
| +CH5 | alkanium ions |
| CH5B | | |
| CH5B | spectator mechanism |
| CH5N3O | | |
| NH2NHC(=O)NH2 | semicarbazones |
| CH5P | | |
| CH3PH2 | phosphines |
| CIN | | |
| ICN | pseudohalogens |
| CKN | | |
| KCN | order-disorder transition |
| KCN | order-disorder transition |
| CKNO | | |
| KOCN | cyanates |
| CN | | |
| CN− | prochirality |
| –C≡N | characteristic group in organic nomenclature |
| C–N | ligases (synthetases) |
| –CN | carbonitriles |
| C–N | lyases |
| C–N | hydrolases |
| CN− | order-disorder transition |
| CN− | pseudohalogens |
| CN− | interfering substance
in electroanalytical chemistry |
| CNNa | | |
| NaCN | cyanides |
| CNNaO | | |
| Na+[–C≡N+O−] | fulminates |
| CNO | | |
| –NCO | characteristic group in organic nomenclature |
| CNS | | |
| SCN− | pseudohalogens |
| N≡C–S− | organoheteryl groups |
| CNS2 | | |
| (SCN)2 | pseudohalogens |
| CO | | |
| C=O | carbonyl compounds |
| CO | isoelectronic |
| CO | air pollutant |
| C–O | lyases |
| –C(=O)– | quinones |
| CO | oxocarbons |
| C–O | hydrolases |
| C–O | ligases (synthetases) |
| C=O | oxo compounds |
| CO2 | | |
| CO2 | carbon dioxide laser (CO2
laser) |
| CO2 | sublimation |
| O=C=O | heterocumulenes |
| CO2 | photophosphorylation |
| CO2 | laser |
| CO2 | photosynthesis |
| CO2 | photosynthesis |
| CO2 | greenhouse effect in atmospheric chemistry |
| CO2 | reduced species |
| CO2 | sanitary land fill |
| CO2 | molecular laser |
| CO2 | acid rain in atmospheric chemistry |
| CO2 | sublimation |
| CO2 | cryogenic sampling |
| O=C=O | oxocarbons |
| CO2 | carbon dioxide laser (CO2
laser) |
| CO2 | composition of pure air in atmospheric chemistry |
| CO2 |
CO2
laser |
| CS | | |
| C–S | ligases (synthetases) |
| CY | | |
| C–Y | bisecting conformation (eclipsing conformation) |
| C2 | | |
| C–C | hydrolases |
| C–C | eclipsing strain |
| C–C | orbital symmetry |
| C–C | symbiosis |
| C–C | lyases |
| C–C | ligases (synthetases) |
| C2H | | |
| HC≡C− | carbanion |
| C2HNa | | |
| NaC≡CH | acetylides |
| C2H2 | | |
| C2H2 | flow rate
in flame emission and absorption spectrometry |
| H2C=C: | vinylidenes |
| –CH=CH– | heteroarenes |
| C2H2 | local fraction atomized,
 , ,
 in flame emission and absorption spectrometry
in flame emission and absorption spectrometry |
| H2C=C: | carbenes |
| C2H2O | | |
| CH2=C=O | heterocumulenes |
| CH2=C=O | isoelectronic |
| C2H3 | | |
| CH2=CH– | vinylic groups |
| C2H3AgO2 | | |
| C2H3AgO2 | molecular rearrangement |
| C2H3Cl | | |
| C2H3Cl | isodesmic reaction |
| :Cl–CH=CH2 | conjugated system (conjugation) |
| C2H3ClO | | |
| C2H3ClO | acyl halides |
| C2H3Cl3 | | |
| C2H3Cl3 | isodesmic reaction |
| C2H3N | | |
| CH3C≡N | cyanides |
| C2H3NS | | |
| CH3SC≡N | thiocyanates |
| C2H3O | | |
| CH3C(=O)– | organyl groups |
| C2H3O2 | | |
| CH3CO2− | Brønsted base |
| –CH2CO2H | prochirality |
| C2H3O2 | proton transfer reaction |
| C2H3O2 | nucleophilic catalysis |
| C2H3O2 | ionization |
| C2H4 | | |
| C2H4 | hydration |
| C2H4 | hyperconjugation |
| C2H4 | attachment |
| C2H4 | ene reaction |
| C2H4 | reactive adsorption |
| +CH2–+CH2 | dicarbenium ions |
| C2H4 | disproportionation |
| –CH2–CH2– | meso structures in polymers |
| C2H4 | sorptive insertion in surface catalysis |
| C2H4 | isodesmic reaction |
| C2H4Cl2 | | |
| C2H4Cl2 | isodesmic reaction |
| C2H4Cl2NO | | |
| (ClCH2)2N–O. | nitroxides |
| C2H4Cl2NO | aminoxyl radicals |
| C2H4Cl2O | | |
| C2H4Cl2O | α-addition (alpha-addition) |
| C2H4Cl3Pt | | |
| [PtCl3(CH2=CH2)]− | chelation |
| C2H4F | | |
| C2H4F | hyperconjugation |
| C2H4N | | |
| CH3–CH=N. | iminyl radicals |
| C2H4O | | |
| C2H4O | stoichiometry |
| C2H4O2 | | |
| C2H4O2 | proton transfer reaction |
| CH3CO2H | Brønsted acid |
| C2H4O2 | ionization |
| C2H4O2 | nucleophilic catalysis |
| C2H4O2 | catalytic hydrogenolysis |
| CH3CO2H | prochirality |
| C2H4O2 | leaving group |
| C2H4O3 | | |
| CH3C(=O)OOH | per acids |
| CH3C(=O)OOH | peroxy acids |
| C2H5 | | |
| C2H5 | disproportionation |
| C2H5 | disproportionation |
| CH3CH2+ | carbenium ion |
| CH3CH2– | organyl groups |
| C2H5Br | | |
| 13C2H581Br+. | isotopic molecular ion |
| 13CCH5Br+. | isotopic molecular ion |
| C2H581Br+. | isotopic molecular ion |
| C2H579Br | molecular ion in mass spectrometry |
| C2H5BrO | | |
| BrCH2CH2OH | halohydrins |
| C2H5Cl | | |
| C2H5Cl | nucleophile (nucleophilic) |
| C2H5N | | |
| MeN=CH2 | heteroalkenes |
| C2H5NO2 | | |
| H3N+CH2C(=O)O− | zwitterionic compounds/zwitterions |
| C2H5N3O2 | | |
| H2NNHC(=O)C(=O)NH2 | semioxamazones |
| C2H6 | | |
| C2H6 | dimerization |
| CH313CH3 | principal ion in mass spectrometry |
| C2H6 | catalytic hydrogenolysis |
| C2H6 | disproportionation |
| C2H6 | catalytic hydrogenolysis |
| C2H6 | catalytic hydrogenolysis |
| C2H6 | pseudorotation |
| C2H6CuLi | | |
| Li+[CuMe2]− | organometallic compounds |
| C2H6NO | | |
| C2H6NO | aminoxides |
| C2H6N2 | | |
| C2H6N2 | carboxamidines |
| C2H6N2 | isoelectronic |
| C2H6N4S2 | | |
| H2NC(=NH)SSC(=NH)NH2 | formamidine disulfides |
| C2H6O | | |
| C2H6O | hydration |
| CH3CH2OH | constitutional isomerism |
| CH3–CH[2H]–OH | singly labelled |
| CH3OCH3 | constitutional isomerism |
| CH3CH2OH | prochirality |
| CH3–CH2–[18O][2H] | mixed labelled |
| C2H6O2 | | |
| HOCH2CH2OH | glycols |
| C2H6S | | |
| MeCH2SH | thiols |
| C2H7 | | |
| [C2H7]+ | alkanium ions |
| C2H7As | | |
| CH3CH2AsH2 | arsines |
| C2H7AsO2 | | |
| C2H7AsO2 | arsinic acids |
| C2H7NS | | |
| C2H5SNH2 | sulfenamides |
| CH3CH2SNH2 | amides |
| C2H7S | | |
| (CH3)2S+H | onium compounds |
| C2H10O3Si3 | | |
| C2H10O3Si3 | cyclosiloxanes |
| C2N | | |
| (CN)2 | pseudohalogens |
| C3 | | |
| C=C=C | chirality axis |
| C3H3AsO | | |
| (CH3)3As=O | arsine oxides |
| C3H3Bi | | |
| (CH3)3Bi | bismuthines |
| C3H3N | | |
| CH2=CH–C≡N | conjugated system (conjugation) |
| C3H4 | | |
| H2C=CHC:H | vinyl carbenes |
| H2C=CHCH: | carbenes |
| C3H5 | | |
| CH2=CHCH2 | allylic groups |
| C3H5 | delocalization |
| C3H5 | delocalization |
| H2C=CHCH2+ | allylic intermediates |
| CH3CH2C… | alkylidynes |
| C3H5NO | | |
| HOCH2CH2C≡N | cyanohydrins |
| C3H5NO | lactams |
| C3H5NO3 | | |
| C3H5NO3 | amic acids |
| C3H5NO3S | | |
| C3H5NO3S | imides |
| C3H5NSe | | |
| CH3CH2SeCN | selenocyanates |
| C3H5O | | |
| C3H5O | abstraction |
| C3H5O | contributing structure |
| C3H5O | contributing structure |
| C3H6 | | |
| CH3CH2CH: | alkylidenes |
| H2C.–CH2–C.H2 | diradicals |
| –CH2CH2CH2– | hydrocarbylene groups |
| +CH2CH2–+CH2 | dicarbenium ions |
| C3H6 | ene reaction |
| C3H6 | corrinoids (cobalamines, corphyrins, corrins, vitamin B12
compounds)
|
| C3H6.+ | radical ion |
| C3H6O | | |
| C3H6O | dimerization |
| C3H6O | dimerization |
| C3H6O | fragmentation |
| C3H6O | Paterno–Büchi reaction |
| C3H6O | isoelectronic |
| C3H6O | proton transfer reaction |
| C3H6O | abstraction |
| C3H6O | epoxy compounds |
| C3H6S | | |
| CH3CH2C(=S)H | thioaldehydes |
| C3H7 | | |
| CH3CH2CH2+ | carbenium ion |
| CH3CH2C.H2 | alkyl radicals |
| C3H7Br | | |
| C3H7Br | molecular rearrangement |
| C3H7ClO | | |
| ClCH2CH2CH2OH | halohydrins |
| C3H7N | | |
| EtCH=NH | aldimines |
| C3H7O | | |
| C3H7O | fragmentation |
| C3H7O | proton transfer reaction |
| C3H8 | | |
| C3H8 | catalytic hydrogenolysis |
| C3H8O2S | | |
| C2H5S(=O)2CH3 | sulfones |
| (CH3)2CHS(=O)OH | sulfinic acids |
| C3H9ClP | | |
| Cl(CH3)3P+ | onium compounds |
| C3H9ClS | | |
| [(CH3)3S]+Cl− | sulfonium compounds |
| C3H9N | | |
| C3H9N | leaving group |
| NMe3 | leaving group |
| C3H9NO | | |
| (CH3)3N+–O− | zwitterionic compounds/zwitterions |
| C3H9NO2S | | |
| C3H9NO2S | amides |
| C3H10N | | |
| C3H10N | ammoniumyl radical ions |
| C3O2 | | |
| O=C=C=C=O | heterocumulenes |
| O=C=C=C=O | oxocarbons |
| C4H4N2 | | |
| C4H4N2 | pyrimidine bases |
| C4H4N2 | heteroarenes |
| C4H4O2 | | |
| C4H4O2 | isolated double bonds |
| HO[CH2]4OH | glycols |
| C4H4S | | |
| C4H4S | heteroarenes |
| C4H5N | | |
| C4H5N | indicated hydrogen |
| C4H5NO2 | | |
| C4H5NO2 | imides |
| C4H6 | | |
| C4H6 | addition reaction |
| C4H6 | attachment |
| CH3–CH=C=CH2 | dienes |
| CH2=CH–CH=CH2 | conjugated system (conjugation) |
| C4H6 | s-cis, s-trans |
| C4H6 | cheletropic reaction |
| CH2=CH–CH=CH2 | dienes |
| CH2=C(C.H2)2 | trimethylenemethanes |
| C4H6 | s-cis, s-trans |
| C4H6Br2 | | |
| C4H6Br2 | addition reaction |
| C4H6Br2 | addition reaction |
| C4H6O | | |
| C4H6O | oxa-di-π-methane rearrangement |
| C4H6O | oxa-di-π-methane rearrangement |
| C4H6O2S | | |
| C4H6O2S | cheletropic reaction |
| C4H6O3 | | |
| C4H6O3 | nucleophilic catalysis |
| C4H6O3 | acid anhydrides |
| C4H6O4 | | |
| C4H6O4 | oxidative coupling |
| C4H6O6 | | |
| C4H6O6 | meso-compound |
| C4H7Br | | |
| C4H7Br | allylic substitution reaction |
| C4H7ClO2Zn | | |
| ClZnCH2C(=O)OEt | organometallic compounds |
| C4H7NO | | |
| (CH3)2C(OH)C≡N | cyanohydrins |
| C4H8 | | |
| C4H8 | hyperconjugation |
| C4H8 | cycloalkanes |
| C4H8 | addition reaction |
| C4H8 | anti |
| C4H8Br2 | | |
| C4H8Br2 | anti |
| C4H8O | | |
| CH3CH2COCH3 | prochirality |
| C4H8O | allylic substitution reaction |
| C4H8O | multiply labelled |
| C4H8O | allylic substitution reaction |
| C4H8S | | |
| CH3C(=S)CH2CH3 | thioketones |
| C4H9 | | |
| C4H9 | hyperconjugation |
| C4H9Br | | |
| C4H9Br | addition reaction |
| C4H9FO | | |
| C4H9FO | diastereotopic |
| C4H9FO | diastereotopic |
| C4H9N | | |
| C4H9N | pre-equilibrium (prior equilibrium) |
| C4H9O | | |
| C4H9O | fragmentation |
| C4H10 | | |
| C4H10 | catalytic hydrogenolysis |
| C4H10 | catalytic hydrogenolysis |
| C4H10 | catalytic hydrogenolysis |
| C4H10Mg | | |
| Et2Mg | organometallic compounds |
| C4H10N | | |
| C4H10N | pre-equilibrium (prior equilibrium) |
| C4H10O | | |
| CH3CHOHCH2CH3 | prochirality |
| C4H10O | diastereotopic |
| CH3CH2OCH2CH3 | ethers |
| CH3CH2CHOHCH3 | prochirality |
| C4H10O3 | | |
| HC(OCH3)3 | ortho esters |
| C4H11N | | |
| (CH3)3N+–C−H2 | ylides |
| C4H11NO | | |
| C4H11NO | pre-equilibrium (prior equilibrium) |
| C4H11O | | |
| C4H11O | fragmentation |
| C4H11O3PS | | |
| C4H11O3PS | betaines |
| C4H12N2 | | |
| C4H12N2 | amine imides |
| C4H12Si | | |
| SiMe4 | chemical shift,  in NMR in NMR |
| SiMe4 | chemical shift,  in NMR in NMR |
| SiMe4 | chemical shift,  in NMR in NMR |
| C4H13NO | | |
| [(CH3)4N]+OH− | quaternary ammonium compounds |
| C4O4 | | |
| C4O4 | oxocarbons |
| C5 | | |
| C5 | terpenes |
| C5H3BrN4 | | |
| C5H3BrN4 | tele-substitution |
| C5H4 | | |
| C5H4 | electrocyclic reaction |
| C5H4N2O | | |
| C5H4N2O | topomerization |
| C5H4N2O | topomerization |
| C5H4N4 | | |
| C5H4N4 | purine bases |
| C5H5N | | |
| C5H5N | nucleophilic catalysis |
| C5H5N+ | organoheteryl groups |
| C5H5N | nucleophilic catalysis |
| C5H5NO | | |
| C5H5NO | tautomerism |
| C5H5NO | tautomerism |
| C5H5NOS | | |
| C5H5NOS | penems |
| C5H5N5 | | |
| C5H5N5 | tele-substitution |
| C5H5OP | | |
| C5H5OP | indicated hydrogen |
| C5H5P | | |
| C5H5P | indicated hydrogen |
| C5H6 | | |
| C5H6 | electrocyclic reaction |
| C5H7NOS | | |
| C5H7NOS | penams |
| C5H7O2 | | |
| C5H7O2 | carbanion |
| C5H7O2 | carbanion |
| C5H8 | | |
| C5H8 | sigmatropic rearrangement |
| [CH2=C(CH3)CH=CH2] | terpenes |
| C5H8 | di-π-methane rearrangement |
| C5H8 | sigmatropic rearrangement |
| C5H8 | di-π-methane rearrangement |
| C5H8OS2 | | |
| CH3C(=O)SC(=S)CH2CH3 | thioanhydrides |
| C5H8O3 | | |
| HC(=O)CH2CH2CH2C(=O)OH | oxo carboxylic acids |
| C5H8O5 | | |
| HO2CCH2CHOHCH2CO2H | prochirality |
| C5H8O7 | | |
| C5H8O7 | pseudo-asymmetric carbon atom |
| C5H8O7 | pseudo-asymmetric carbon atom |
| C5H9Br | | |
| C5H9Br | molecular rearrangement |
| C5H9NO2 | | |
| C5H9NO2 | imino acids |
| C5H9O | | |
| C5H9O | ambident |
| C5H9O | ambident |
| C5H10 | | |
| C5H10 | ene reaction |
| C5H10 | envelope conformation |
| C5H10O | | |
| C5H10O | prochirality |
| C5H10O | molecular rearrangement |
| C5H10O2 | | |
| C5H10O2 | molecular rearrangement |
| C5H10O4 | | |
| C5H10O4 | monosaccharides |
| C5H10O4 | monosaccharides |
| C5H11NO2 | | |
| C5H11NO2 | betaines |
| C5H12 | | |
| Me4C | symbiosis |
| C5H12O4 | | |
| C(OCH3)4 | ortho esters |
| C5H12O5 | | |
| C5H12O5 | meso-compound |
| C5H14NO4P | | |
| Me3N+–CH2CH2–OP(=O)(OH)O− | phospho |
| C5H15Ta | | |
| TaMe5 | homoleptic |
| C6H4 | | |
| C6H4 | aryne |
| C6H4NS2 | | |
| C6H4NS2 | Herz compounds |
| C6H4O2 | | |
| C6H4O2 | quinhydrones |
| C6H4O2 | quinones |
| C6H5 | | |
| C6H5 | aryl cations |
| C6H5AsCa | | |
| CaAsPh | arsenides |
| C6H5I | | |
| C6H5I | isotopic scrambling |
| C6H5KN2O | | |
| PhN=NO−K+ | diazoates |
| C6H5NO2 | | |
| C6H5NO2 | electrophile (electrophilic) |
| C6H5N2 | | |
| C6H5N2 | isotopic scrambling |
| C6H5N2 | isotopic scrambling |
| C6H5N2 | isotopic scrambling |
| PhN+≡N | diazonium salts |
| C6H5N3 | | |
| PhN3 | azides |
| C6H5Y | | |
| C6H5Y | Hammett equation (Hammett relation) |
| C6H6 | | |
| C6H6.− | radical ion |
| C6H6.+ | radical ion |
| C6H6 | fulvenes |
| C6H6 | Kekulé structure (for aromatic compounds) |
| C6H6 | catalytic hydrogenolysis |
| C6H6 | Kekulé structure (for aromatic compounds) |
| C6H6 | electrophile (electrophilic) |
| C6H6NNa | | |
| C6H6NNa | anilides |
| C6H6N2 | | |
| C6H6N2 | quinonimines (quinone imines) |
| C6H6N2O | | |
| PhN=NOH | diazoates |
| C6H6O | | |
| C6H6O | valence tautomerization |
| C6H6O | arene epoxides |
| C6H6O | valence tautomerization |
| C6H6OS | | |
| PhSOH | sulfenic acids |
| C6H6O2 | | |
| C6H6O2 | quinhydrones |
| C6H6O4 | | |
| C6H6O4 | cycloaddition |
| C6H6O4S | | |
| PhS(=O)2OOH | peroxy acids |
| C6H7 | | |
| C6H7+ | arenium ions |
| C6H7N | | |
| C6H5NH2 | transformation |
| C6H7N | isotopic scrambling |
| C6H7N | isotopic scrambling |
| C6H8 | | |
| C6H8 | electrocyclic reaction |
| C6H8 | electrocyclic reaction |
| C6H8N | | |
| C6H8N | ammoniumyl radical ions |
| C6H8NO2P | | |
| C6H8NO2P | amides |
| C6H8N2S | | |
| PhS(=NH)NH2 | sulfinamidines |
| C6H9Br | | |
| C6H9Br | anti |
| C6H9NOS | | |
| C6H9NOS | cephams |
| C6H9N3O2 | | |
| C6H9N3O2 | pros in histidine nomenclature |
| C6H9Sb | | |
| (CH2=CH)3Sb | stibines |
| C6H10 | | |
| C6H10 | degenerate rearrangement |
| C6H10 | attachment |
| C6H10 | half-chair |
| C6H10 | degenerate rearrangement |
| C6H10BrF | | |
| C6H10BrF | anti |
| C6H10Br2NiP2 | | |
| Ni[(CH3)2PCH2CH2P(CH3)2]Br2 | κ (kappa) in inorganic nomenclature |
| C6H10O3 | | |
| C6H10O3 | tautomerism |
| C6H10O3 | tautomerism |
| C6H10O4 | | |
| C6H10O4 | enoses |
| C6H10O7 | | |
| C6H10O7 | ketoaldonic acids |
| C6H10O7 | uronic acids |
| C6H11 | | |
| C6H11 | carbenes |
| C6H11BrO5 | | |
| C6H11BrO5 | anomeric effect |
| C6H11BrO5 | anomeric effect |
| C6H11NO | | |
| C6H11NO | prochirality |
| C6H11NO | lactams |
| C6H11NO | prochirality |
| C6H11S | | |
| C6H11S | sulfonium compounds |
| C6H12 | | |
| C6H12 | Paterno–Büchi reaction |
| C6H12 | chair, boat, twist |
| C6H12 | oxidative addition |
| C6H12 | cyclo- |
| C6H12 | chair, boat, twist |
| C6H12 | chair, boat, twist |
| C6H12O2 | | |
| C6H12O2 | dimerization |
| C6H12O3 | | |
| C6H12O3 | acetonides |
| C6H12O5 | | |
| C6H12O5 | α (alpha), β (beta) |
| C6H12O6 | | |
| C6H12O6 | aldoses |
| C6H12O6 | aldoses |
| C6H12O6 | aldoses |
| C6H12O6 | Haworth representation |
| C6H12O6 | ketoses |
| C6H12O6 | Haworth representation |
| C6H12O6 | α (alpha), β (beta) |
| C6H12O6 | ketoses |
| C6H12O6 | Haworth representation |
| C6H12O6 | Haworth representation |
| C6H12O7 | | |
| C6H12O7 | aldonic acids |
| C6H13NO5 | | |
| C6H13NO5 | amino sugars |
| C6H14 | | |
| C6H14 | cyclo- |
| C6H14LiN | | |
| C6H14LiN | amides |
| C6H14N | | |
| C6H14N | abstraction |
| C6H14O6 | | |
| C6H14O6 | Haworth representation |
| C6H14O6 | Haworth representation |
| C6H15B | | |
| Et3B | organometallic compounds |
| C6H15N | | |
| C6H15N | abstraction |
| C6N4 | | |
| C6N4 | π-adduct |
| C7H4ClO2 | | |
| C7H4ClO2 | isodesmic reaction |
| C7H4O3S2 | | |
| C7H4O3S2 | cyclic acid anhydrides (cyclic anhydrides) |
| C7H5ClO2 | | |
| C7H5ClO2 | isodesmic reaction |
| C7H5NO | | |
| PhOCN | cyanates |
| C7H5O2 | | |
| C7H5O2 | isodesmic reaction |
| C7H6FeO2S | | |
| [Fe(CO)3(C4H6SO)] | η (eta or hapto) in inorganic nomenclature |
| C7H6N | | |
| C7H6N | nitrilium ions |
| C7H6N | nitrilium ions |
| C7H6O | | |
| C7H6O | quinomethanes |
| C7H6O | tropones |
| C7H6OS | | |
| PhC(=S=O)H | sulfines |
| C7H6O2 | | |
| C7H6O2 | tropolones |
| C7H6O2 | isodesmic reaction |
| C7H7 | | |
| C7H7 | annulenylidenes |
| C7H7+ | tropylium ions |
| C7H7 | benzylic intermediates |
| C7H7 | hyperconjugation |
| (C7H72+) | mass-to-charge ratio,
 in mass spectrometry
in mass spectrometry |
| C7H7 | common-ion effect (on rates) |
| C7H7 | common-ion effect (on rates) |
| C7H7Br | | |
| C7H7Br | leaving group |
| C7H7BrO | | |
| C7H7BrO | cine-substitution |
| C7H7Cl | | |
| C7H7Cl | common-ion effect (on rates) |
| C7H7NO | | |
| C7H7NO | amides |
| C7H7NS | | |
| PhC(=S)NH2 | thio |
| C7H8 | | |
| C7H8 | catalytic hydrogenolysis |
| C7H8 | hyperconjugation |
| C7H8 | catalytic hydrogenolysis |
| C7H8 | catalytic dehydrocyclization |
| C7H8 | cycloaddition |
| C7H8 | tropilidenes |
| C7H8 | catalytic hydrogenolysis |
| C7H8 | tropilidenes |
| C7H8NO | | |
| C7H8NO | nucleophilic catalysis |
| C7H8NO | nucleophilic catalysis |
| C7H8O | | |
| C7H8O | common-ion effect (on rates) |
| C7H9N | | |
| C7H9N | catalytic hydrogenolysis |
| C7H9NO | | |
| C7H9NO | cine-substitution |
| C7H9NOS | | |
| PhS(=O)NHCH3 | sulfinamides |
| C7H9NO2S | | |
| PhS(=O)2NHCH3 | sulfonamides |
| C7H10BrF | | |
| C7H10BrF | endo, exo, syn, anti |
| C7H10BrF | endo, exo, syn, anti |
| C7H12 | | |
| C7H12 | anti |
| C7H12ClN | | |
| C7H12ClN | acyl halides |
| C7H14N2 | | |
| C7H14N2 | carboxamidines |
| C7H14O | | |
| C7H14O | Norrish type II photoreaction |
| C7H14O | Norrish type II photoreaction |
| C7H14O6 | | |
| C7H14O6 | anomeric effect |
| C7H14O6 | anomeric effect |
| C7H16 | | |
| C7H16 | catalytic dehydrocyclization |
| C8H4O | | |
| C8H4O | heteroarynes |
| C8H5NO | | |
| C8H5NO | cyanides |
| C8H6CrO | | |
| [Cr(CO)4(C4H6)] | η (eta or hapto) in inorganic nomenclature |
| C8H6S2 | | |
| C8H6S2 | isolated double bonds |
| C8H8 | | |
| C8H8 | chain transfer |
| C8H8 | chain reaction |
| C8H8 | alternant |
| C8H8 | quinomethanes |
| C8H8 | polyhedranes |
| C8H8 | chain transfer |
| C8H8ClNO | | |
| C8H8ClNO | molecular rearrangement |
| C8H8O4 | | |
| C8H8O4 | polyketides |
| C8H8O4S | | |
| C8H8O4S | acid anhydrides |
| C8H9 | | |
| (C8H9+) | homoaromatic |
| C8H9ClO | | |
| PhCH(OH)CH2Cl | halohydrins |
| C8H9N | | |
| PhCH=NMe | aldimines |
| C8H9NO | | |
| C8H9NO | molecular rearrangement |
| C6H5NHCOCH3 | transformation |
| C8H9NO | molecular rearrangement |
| C8H9NO | anilides |
| C8H10S | | |
| C8H10S | leaving group |
| C8H11ClO | | |
| C8H11ClO | α (alpha), β (beta) |
| C8H11NOS | | |
| (CH3)2S(=O)=NPh | sulfoximides |
| C8H12 | | |
| C8H12 | propellanes |
| C8H12 | electrocyclic reaction |
| C8H12 | electrocyclic reaction |
| C8H12N | | |
| C8H12N | aminium ions |
| C8H12O5 | | |
| C8H12O5 | tautomerism |
| C8H12O5 | tautomerism |
| C8H14 | | |
| C8H14 | planar chirality |
| C8H14O4 | | |
| C8H14O4 | monosaccharides |
| C8H16 | | |
| C8H16 | extrusion transformation |
| C8H16 | crown conformation |
| C8H16 | tub conformation |
| C8H16N2 | | |
| C8H16N2 | extrusion transformation |
| C8H18 | | |
| C8H18 | catalytic hydrogenolysis |
| C8H18O | | |
| C8H18O | zig-zag projection |
| C8H20N | | |
| (CH3CH2)4N+ | onium compounds |
| C9H5N | | |
| C9H5N | heteroarynes |
| C9H6O2 | | |
| C9H6O2 | coumarins |
| C9H6O2 | isocoumarins |
| C9H8N2 | | |
| C9H8N2 | dipyrrins |
| C9H9Cl3 | | |
| C9H9Cl3 | chain transfer |
| C9H9Cl3 | chain transfer |
| C9H10O | | |
| C9H10O | molecular rearrangement |
| C9H10O | molecular rearrangement |
| C9H10O | molecular rearrangement |
| C9H10O2 | | |
| C9H10O2 | catalytic hydrogenolysis |
| C9H10O2 | leaving group |
| C9H12 | | |
| C9H12 | π-adduct |
| C9H12S | | |
| (CH3)2S+–C−HPh | ylides |
| (CH3)2S=CHPh | ylides |
| C9H14 | | |
| C9H14 | Bredt's rule |
| C9H14 | Bredt's rule |
| C9H14O4 | | |
| C9H14O4 | tautomerism |
| C9H16 | | |
| C9H16 | spiro compounds |
| C9H16O2 | | |
| C9H16O2 | tritactic polymer |
| C9H18O | | |
| C9H18O | Paterno–Büchi reaction |
| C9H19NO4 | | |
| C9H19NO4 | glycosylamines |
| C10 | | |
| C10 | terpenes |
| C10 | monoterpenoids |
| C10H6 | | |
| C10H6 | dehydroarenes |
| C10H8 | | |
| C10H8 | alternant |
| C10H8 | fulvalenes |
| C10H8O | | |
| C10H8O | phenols |
| C10H10 | | |
| C10H10 | polyquinanes (polyquinenes) |
| C10H14NO | | |
| C10H14NO | amidium ions |
| C10H15NS | | |
| (C2H5)2S=NPh | sulfimides |
| C10H16 | | |
| C10H16 | electrocyclic reaction |
| C10H16 | electrocyclic reaction |
| C10H16 | ring assembly |
| C10H18 | | |
| C10H18 | cis-trans isomers |
| C10H18 | cis-trans isomers |
| C10H20 | | |
| C10H20 | iridoids |
| C11H6O3 | | |
| C11H6O3 | furocoumarins |
| C11H6O3 | furocoumarins |
| C11H12O4 | | |
| C11H12O4 | acylals |
| C12H8 | | |
| C12H8 | ring assembly |
| C12H8Cl2 | | |
| C12H8Cl2 | meso-compound |
| C12H10 | | |
| C12H10 | ring assembly |
| C12H10N2 | | |
| PhN=NPh | azo compounds |
| C12H10N2O | | |
| C12H10N2O | azoxy compounds |
| C12H10OS | | |
| Ph2S=O | sulfoxides |
| C12H10O5S2 | | |
| PhS(=O)2OS(=O)2Ph | sulfonic anhydrides |
| C12H12N2S | | |
| Ph2S(=NH)2 | sulfonediimines |
| C12H14 | | |
| C12H14 | anti |
| C12H24O6 | | |
| C12H24O6 | crown |
| C12O9 | | |
| C12O9 | oxocarbons |
| C13H10O | | |
| Ph2C–O.− | radical ion |
| C13H12O2 | | |
| C13H12O2 | bisphenols |
| C13H13N3 | | |
| PhN=N–NPhMe | diazoamino compounds |
| C13H13OP | | |
| (Ph)2POCH3 | esters |
| C13H14 | | |
| C13H14 | spiro compounds |
| C13H14O4 | | |
| C13H14O4 | cycloaddition |
| C13H16O7 | | |
| C13H16O7 | glycosides |
| C14H10 | | |
| C14H10 | acenes |
| C14H10 | quinarenes |
| C14H10O | | |
| C14H10O | epoxy compounds |
| C14H10OS2 | | |
| C14H10OS2 | acid anhydrides |
| C14H10O9 | | |
| C14H10O9 | depsides |
| C14H12 | | |
| C14H12 | cycloaddition |
| C14H12N6O3 | | |
| C14H12N6O3 | folates |
| C14H24 | | |
| C14H24 | propellanes |
| C15 | | |
| C15 | sesquiterpenoids |
| C15 | terpenes |
| C15H10O2 | | |
| C15H10O2 | flavonoids (isoflavonoids and neoflavonoids) |
| C15H10O2 | flavonoids (isoflavonoids and neoflavonoids) |
| C15H10O2 | flavonoids (isoflavonoids and neoflavonoids) |
| C15H11O | | |
| C15H11O | anthocyanidins |
| C15H16N | | |
| (p-Me2NC6H4)2CHPh | leuco bases |
| C16H10 | | |
| C16H10 | ortho- and peri-fused (polycyclic compounds) |
| C16H14O | | |
| PhC(=O)CH=C(CH3)Ph | dypnones |
| C16H16 | | |
| C16H16 | cyclophanes |
| C17H17Cl3 | | |
| C17H17Cl3 | chain transfer |
| C17H21NO4 | | |
| C17H21NO4 | pseudo-asymmetric carbon atom |
| C17H21N2 | | |
| C17H21N2 | cyanine dyes |
| C17H28 | | |
| C17H28 | steroids |
| C18H12 | | |
| C18H12 | ortho-fused (polycyclic compounds) |
| C18H14 | | |
| C18H14 | quinarenes |
| C18H34O9 | | |
| C18H34O9 | in-out isomerism |
| C18H34O9 | in-out isomerism |
| C18H34O9 | in-out isomerism |
| C18H36N2O6 | | |
| C18H36N2O6 | cryptand |
| C19H12 | | |
| C19H12 | ortho- and peri-fused (polycyclic compounds) |
| C19H14N4 | | |
| C19H14N4 | tetrapyrroles |
| C19H14O5S | | |
| C19H14O5S | sulfonphthaleins |
| C19H15 | | |
| C19H15 | fragmentation |
| C19H17P | | |
| Ph3P+–C−H2 | ylides |
| Ph3P+=CH2 | ylides |
| C19H22N4 | | |
| C19H22N4 | corrinoids (cobalamines, corphyrins, corrins, vitamin B12
compounds)
|
| C19H30 | | |
| C19H30 | cyclo- |
| C19H32 | | |
| C19H32 | seco- |
| C19H32 | abeo- |
| C19H32 | abeo- |
| C19H32 | abeo- |
| C19H32 | cyclo- |
| C19H32O | | |
| C19H32O | α (alpha), β (beta) |
| C19H34 | | |
| C19H34 | seco- |
| C20 | | |
| C20 | terpenes |
| C20 | icosanoids |
| C20 | prostaglandins |
| C20 | diterpenoids |
| C20 | leukotrienes |
| C20H12O5 | | |
| C20H12O5 | xanthene dyes |
| C20H14N4 | | |
| C20H14N4 | porphyrins |
| C20H14O4 | | |
| C20H14O4 | phthaleins |
| C20H16O2 | | |
| C20H16O2 | fragmentation |
| C20H19OP | | |
| C20H19OP | betaines |
| C20H20O5 | | |
| C20H20O5 | lignans |
| C20H38O2 | | |
| C20H38O2 | prostaglandins |
| C22H23ClN2O8 | | |
| C22H23ClN2O8 | tetracyclines |
| C22H26O6 | | |
| C22H26O6 | lignans |
| C23H22O6 | | |
| C23H22O6 | rotenoids |
| C25 | | |
| C25 | sesterterpenoids |
| C25 | terpenes |
| C26H16 | | |
| C26H16 | helicity |
| C26H16 | helicenes |
| C27H54N2 | | |
| C27H54N2 | in-out isomerism |
| C27H54N2 | in-out isomerism |
| C27H54N2 | in-out isomerism |
| C30 | | |
| C30 | terpenes |
| C30 | triterpenoids |
| C30H42O | | |
| C30H42O | carotenoids |
| C38H24Cl4 | | |
| C38H24Cl4 | biradical |
| C40 | | |
| C40 | tetraterpenoids |
| C40 | terpenes |
| C40 | carotenoids |
| C40H56 | | |
| C40H56 | retro |
| C40H56 | retro |
| C40H56 | carotenoids |
| C40H56O2 | | |
| C40H56O2 | carotenoids |
| C41H29NO | | |
| C41H29NO | Dimroth–Reichardt  parameter parameter |
| Ca | | |
| Ca2+ | selectivity coefficient,
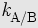 in ion exchange chromatography
in ion exchange chromatography |
| Ca2+ | interfering substance
in electroanalytical chemistry |
| Ca2+ | ionic conductivity |
| CaFeO3 | | |
| CaFeO3 | disproportionation |
| Cd | | |
| Cd | resonance lamp |
| CdHe | | |
| HeCd | ion laser |
| CdHgTe | | |
| HgCdTe | photoconductive detector |
| CdS | | |
| CdS | pressure-induced transition |
| CdS | photoconductive detector |
| CdS | pressure-induced transition |
| Cl | | |
| Cl | common-ion effect (on rates) |
| Cl | chain reaction |
| Cl+ | halonium ions |
| Cl− | conjugate acid–base pair |
| Cl− | gas sensing electrode |
| Cl− | gravimetric method |
| Cl | ionization |
| Cl. | chain reaction |
| Cl | Herz compounds |
| Cl− | gas sensing electrode |
| Cl− | nucleofuge |
| Cl. | radical (free radical) |
| Cl− | selectivity coefficient,
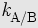 in ion exchange chromatography
in ion exchange chromatography |
| Cl | chain reaction |
| Cl | substitution reaction |
| Cl− | Brønsted base |
| Cl | nucleophile (nucleophilic) |
| –Cl | characteristic group in organic nomenclature |
| Cl | abstraction |
| Cl | photolysis |
| ClCs | | |
| CsCl | dilational (dilatational) transition |
| CsCl | structural transition |
| CsCl | first-order phase transition |
| CsCl | thermally-induced transition |
| CsCl | thermally-induced transition |
| CsCl | first-order phase transition |
| ClH | | |
| HCl | internal filling solution of a glass electrode |
| ClH | abstraction |
| HCl | conjugate acid–base pair |
| HCl | levelling effect |
| HCl | Brønsted acid |
| HCl | oxoacids |
| ClH | molecular rearrangement |
| ClH | reaction path degeneracy |
| ClH | isotope exchange |
| ClH | molecular rearrangement |
| ClH | reaction path degeneracy |
| ClHO | | |
| HOCl | oxoacids |
| ClHO4 | | |
| HClO4 | levelling effect |
| ClH2 | | |
| (H2Cl+) | onium compounds |
| ClH4N | | |
| NH4Cl | structural transition |
| ClK | | |
| KCl | Donnan emf (Donnan potential) |
| KCl | pH |
| KCl | membrane emf |
| ClLiO4 | | |
| LiClO4 | special salt effect |
| ClNa | | |
| NaCl | pressure-induced transition |
| NaCl | thermally-induced transition |
| NaCl | structural transition |
| NaCl | first-order phase transition |
| ClXe | | |
| XeCl | excimer laser |
| Cl2 | | |
| Cl2 | polarizability |
| Cl2 | gas sensing electrode |
| Cl2 | gas sensing electrode |
| 2Cl− | mean activity of an electrolyte in solution |
| Cl2F | | |
| (Cl2F+) | onium compounds |
| Cl2Zn | | |
| ZnCl2 | activated carbon |
| Cl4Rb2Zn | | |
| Rb2ZnCl4 | commensurate–incommensurate transition |
| Co | | |
| Co3+ | spin-state transition |
| CoLaO3 | | |
| LaCoO3 | spin-state transition |
| Cr | | |
| Cr3+ | ruby laser |
| Cr3+ | solid state lasers |
| Cu | | |
| Cu | Penning gas mixture |
| Cu | Penning gas mixture |
| CuI | | |
| CuI | chelation |
| CuMn | | |
| Cu–Mn | spin-glass transition |
| CuZn | | |
| CuZn | second-order transition |
| F | | |
| F+ | halonium ions |
| F | hyperconjugation |
| F | branching ratio |
| F | hydrogen bond |
| FH | | |
| HF | chemical laser |
| HF | branching ratio |
| HF | molecular laser |
| HF | superacid |
| FHO3S | | |
| HSO3F | superacid |
| HSO3F | superacid |
| FH2 | | |
| (H2F+) | onium compounds |
| FKr | | |
| KrF | excimer laser |
| KrF | liquid ion laser |
| F5P | | |
| PF5 | polytopal rearrangement |
| F5Sb | | |
| SbF5 | superacid |
| SbF5 | superacid |
| F6S | | |
| SF6 | air mass in atmospheric chemistry |
| F6U | | |
| UF6 | gaseous diffusion separator
in atmospheric chemistry |
| Fe | | |
| Fe3+ | Fenton reaction |
| Fe | exchange-inversion transition |
| Fe3+ | order-disorder transition |
| Fe3+ | oxidized species |
| Fe3+ | oxidized species |
| Fe+3 | order-disorder transition |
| Fe | oxidized species |
| Fe | oxidized species |
| Fe2+ | oxidized species |
| Fe2+ | Verwey transition |
| Fe3+ | equivalent entity |
| Fe2+ | oxidized species |
| Fe3+ | Verwey transition |
| FeIII | salt form of an ion exchanger |
| FeII | salt form of an ion exchanger |
| Fe2+ | Fenton reaction |
| 57Fe | quadrupole splitting in Mössbauer spectroscopy |
| 57Fe | nuclear quadrupole moment (spectroscopic) |
| FeLiO2 | | |
| LiFeO2 | order-disorder transition |
| LiFeO2 | order-disorder transition |
| FeMo | | |
| Mo–Fe | spin-glass transition |
| FeRh | | |
| FeRh | exchange-inversion transition |
| Fe2O3 | | |
| α-Fe2O3 | Morin transition |
| Fe3O4 | | |
| Fe3+[Fe3+Fe2+]O4 | Verwey transition |
| H | | |
| H | acetals |
| H | Schiff bases (Schiff's bases) |
| H+ | protium |
| H | sulfoxides |
| 2H | deuterium |
| H | nitrones |
| H | hemiacetals |
| 3H | electron capture detector in gas chromatography |
| H+ | gas sensing electrode |
| H+ | general acid–base catalysis |
| H | ketoximes |
| H+ | ligands |
| 2H | deuterium |
| H | trioxides |
| H | sulfenic acids |
| 3H+ | tritium |
| H | extended Hammett equation |
| H | tellurides |
| H | bismuthines |
| H | selenenic acids |
| 2H+ | deuterium |
| H | thioethers |
| 1H | aromatic |
| H+ | protonation constant |
| H+ | equivalent entity |
| H+ | electrofuge |
| 3H | tritium |
| H | sulfides |
| H | ortho esters |
| H+ | Haber–Weiss reaction |
| H | esters |
| H | phosphines |
| H | ketones |
| H+ | specific acid–base catalysis |
| H+ | Rutherford backscattering (RBS) |
| H | ketals |
| H | dual substituent-parameter equation |
| H | thioacetals |
| H | selenoxides |
| 1H | protium |
| H | enols |
| H | selenides |
| H | ortho esters |
| H+ | catalytic coefficient |
| H | enols |
| H+ | gas sensing electrode |
| H | alkoxyamines |
| H | Hammett equation (Hammett relation) |
| H | bond-dissociation energy,  |
| H | multiply labelled |
| 2H | deuteron |
| H+ | leaving group |
| H | azomethines |
| H | arsines |
| H | thiohemiacetals |
| H | imines |
| 1H | protium |
| H | thioacetals |
| H | imines |
| H | azomethines |
| H | selones |
| H | hydrogen |
| H+ | isoionic |
| H+ | extraction (equilibrium) constant |
| H+ | tautomerism |
| H | hydrazides |
| H | sulfones |
| H | silicones |
| H | branching chain reaction |
| H | thiols |
| H | acetals |
| H | hemiketals |
| H | alkyl groups |
| H | polysulfanes |
| 3H− | tritium |
| H | tautomerism |
| H+ | standard hydrogen electrode |
| H | ketimines |
| H | ethers |
| H+ | ligands |
| H | thioacetals |
| H | selenols |
| H | diazoamino compounds |
| 1H | protium |
| H+ | hydron |
| H | oxime O-ethers |
| H | sulfenyl radicals |
| 1H+ | protium |
| H | Brønsted relation |
| H+ | gas sensing electrode |
| H | stibines |
| H | carbene analogues |
| H | acetals |
| H | urethanes (urethans) |
| H | thioketones |
| H− | hydron |
| H | sulfenamides |
| 1H | chemical shift,  in NMR in NMR |
| H | alkyl groups |
| H | thioketone S-oxides |
| H | hemiaminals |
| H | sulfenyl groups |
| H | extended Hammett equation |
| H | polysulfides |
| 3H | tritium |
| H | hydron |
| H | Hammett equation (Hammett relation) |
| H | sulfenylium ions |
| 2H− | deuterium |
| HI | | |
| HI | levelling effect |
| HLi | | |
| LiH | oxidation state |
| HLi | topochemical reaction |
| HN | | |
| HN: | carbene analogues |
| –NH | anion exchanger |
| –NH– | silazanes |
| HN= | imino acids |
| NH | silasesquiazanes |
| HN: | nitrenes |
| HNO | | |
| =NOH | hydroximic acids |
| HNO2 | | |
| HONO | smog chamber in atmospheric chemistry |
| HON=O | oxoacids |
| HNO3 | | |
| HNO3 | smog chamber in atmospheric chemistry |
| HN3 | | |
| –N=N–NH– | azimines |
| HN3 | azides |
| HO | | |
| OH | phosphoramides |
| –OH | hydroxamic acids |
| OH. | Haber–Weiss reaction |
| HO. | acyl species |
| OH | oxo compounds |
| OH | phosphoramides |
| HO+ | oxylium ions |
| –OH | characteristic group in organic nomenclature |
| OH | erythro structures in a polymer |
| HO | molecular rearrangement |
| HO. | alcohols |
| –OH | cresols |
| –OH | benzylic groups |
| –OH | vinylic groups |
| OH− | specific acid–base catalysis |
| HO | allylic substitution reaction |
| OH. | Haber–Weiss reaction |
| OH− | Haber–Weiss reaction |
| HO+ | acyl species |
| OH | anilides |
| HO | decay rate in atmospheric chemistry |
| OH. | Fenton reaction |
| –OH | peroxy acids |
| OH− | catalytic coefficient |
| OH− | kinetic equivalence |
| –OH | alcohols |
| –OH | hydrazidines |
| HO− | acyl species |
| OH | phosphoglycerides |
| HO | smog chamber in atmospheric chemistry |
| HO | colligation |
| –OH | hydrazides |
| OH− | isoionic |
| OH– | anion exchanger |
| OH | branching chain reaction |
| OH− | Brønsted base |
| –OH | allylic groups |
| HO | pre-equilibrium (prior equilibrium) |
| OH− | general acid–base catalysis |
| HO | substitution reaction |
| OH− | Fenton reaction |
| –OH | sulfenamides |
| HO | anhydro bases |
| HO2 | | |
| –OOH | peroxy acids |
| HO3S | | |
| –SO3H | characteristic group in organic nomenclature |
| HO4S | | |
| HSO4− | Brønsted acid |
| HSO4− | Brønsted base |
| HSi | | |
| Si–H | agostic |
| SiH | silasesquiazanes |
| H2 | | |
| H2 | branching chain reaction |
| H2 | standard hydrogen electrode |
| H2 | reaction path degeneracy |
| H2 | catalytic hydrogenolysis |
| H2 | standard hydrogen electrode |
| H2 | gas sensing electrode |
| H2 | gas sensing electrode |
| H2 | bond energy (mean bond energy) |
| H2 | standard hydrogen electrode |
| H2I | | |
| (H2I+) | onium compounds |
| H2KN | | |
| H2KN | isotopic scrambling |
| H2KO4P | | |
| KH2PO4 | pH |
| H2N | | |
| NH2 | phosphoglycerides |
| NH2 | amides |
| H2N | Lewis acid |
| H2N. | aminyl radicals |
| –NH2 | characteristic group in organic nomenclature |
| (H2N:+) | nitrenium ions |
| (H2N:+) | nitrenium ions |
| H2NO | | |
| H2N–O− | aminoxides |
| –NHOH | hydroxamic acids |
| H2N–O. | nitroxides |
| H2NP | | |
| HP=NH | phosphazenes |
| H2N2 | | |
| HN=NH | azo compounds |
| =NNH2 | hydrazones |
| –NHNH– | hydrazo compounds |
| H2NN: | carbene analogues |
| =NNH2 | hydrazidines |
| H2N2O | | |
| H2NNO | nitrosamides |
| H2N2O2 | | |
| O2NNH2 | nitramines |
| H2N2S | | |
| HN=S=NH | sulfur diimides |
| H2O | | |
| H2O | gaseous diffusion separator
in atmospheric chemistry |
| H2O | sanitary land fill |
| H2O | photosynthesis |
| H2O | Brønsted base |
| H2O | ionization |
| H2O | branching chain reaction |
| H2O | oxidative coupling |
| H2O | supersaturation |
| H2O | pre-equilibrium (prior equilibrium) |
| H2O | common-ion effect (on rates) |
| H2O | oxenium ions |
| H2O | amphiprotic (solvent) |
| H2O | Brønsted acid |
| H2O | temperature lapse rate in atmospheric chemistry |
| H2O | hydration |
| H2O | anhydro bases |
| H2O | composition of pure air in atmospheric chemistry |
| H2O | nucleophilic catalysis |
| H2O | Haber–Weiss reaction |
| H2O2 | | |
| H2O2 | Fenton reaction |
| HOOH | hydroperoxides |
| H2O2 | oxidation state |
| H2O2 | smog chamber in atmospheric chemistry |
| H2O2 | Haber–Weiss reaction |
| H2O2 | Haber–Weiss reaction |
| H2O2S | | |
| HS(=O)OH | sulfinic acids |
| HOSOH | oxoacids |
| H2O3 | | |
| HOOOH | trioxides |
| H2O3P | | |
| –P(=O)(OH)2 | phosphono |
| –P(=O)(OH)2 | phospho |
| –PO3H2 | characteristic group in organic nomenclature |
| H2O3S | | |
| HS(=O)2OH | sulfonic acids |
| H2O4S | | |
| H2SO4 | equivalent entity |
| (HO)2SO2 | oxoacids |
| H2SO4 | amphoteric |
| H2SO4 | Brønsted acid |
| H2SO4 | haze in atmospheric chemistry |
| H2SO4 | reduced species |
| H2SO4 | oxidation state |
| H2S | | |
| H2S | oxidized species |
| H2S | hydrocracking unit |
| H2S | reduced species |
| H2S | oxidation state |
| H2S | air pollutant |
| H2S | sulfanes |
| H2S | sulfenylium ions |
| H2S | sulfenyl radicals |
| SH2 | bonding number |
| H2S | acid-labile sulfur |
| H2S | sulfenyl groups |
| H2S2 | | |
| HS2H | polysulfanes |
| H2Se | | |
| H2Se | selenides |
| H2Si | | |
| H2Si: | silylene |
| H2Si | silylene |
| H2Te | | |
| H2Te | tellurides |
| H3N | | |
| H3N | tele-substitution |
| NH3 | Brønsted acid |
| H3N.+ | ammoniumyl radical ions |
| H3NO | | |
| H2N–OH | hydroxylamines |
| H3NO2 | | |
| H3NO2 | azinic acids |
| H3NO3 | | |
| H3NO3 | azonic acids |
| H3NO3S | | |
| H2NS(=O)2OH | sulfamic acids |
| H3NS | | |
| H2S=NH | sulfimides |
| H3N2 | | |
| H2NN.H | verdazyl radicals |
| –NHNH2 | hydrazidines |
| H3N3 | | |
| NH2N=NH | triazenes |
| H3O | | |
| H3O+ | oxonium ions |
| H3O | ionization |
| H3O+ | Brønsted acid |
| (H3O+) | onium compounds |
| H3OP | | |
| H2POH | phosphinous acids |
| H3O2P | | |
| HP(OH)2 | phosphonous acids |
| H2P(=O)OH | phosphinic acids |
| H3O3P | | |
| P(OH)3 | oxoacids |
| HP(=O)(OH)2 | phosphonic acids |
| H3O4P | | |
| H3PO4 | electrolytic hygrometer |
| H3P | | |
| PH3 | phosphanes |
| PH3 | phosphines |
| H3S | | |
| (H3S+) | onium compounds |
| H3Sb | | |
| SbH3 | stibanes |
| SbH3 | stibines |
| H3Se | | |
| (H3Se+) | onium compounds |
| H3Si | | |
| H3Si– | silyl groups |
| H3Si. | silyl radicals |
| H3Sn | | |
| .SnH3 | radical (free radical) |
| H3Te | | |
| (H3Te+) | onium compounds |
| H4N | | |
| H4N | aminiumyl radical ions |
| (H4N+) | onium compounds |
| H4NP | | |
| H3P=NH | phosphazenes |
| H4NY | | |
| (NH4+)Y− | quaternary ammonium compounds |
| H4N2 | | |
| H2NNH2 | hydrazines |
| H4OSi | | |
| H3SiOH | silanols |
| H4P | | |
| (H4P+) | onium compounds |
| H4Sb | | |
| (H4Sb+) | onium compounds |
| H5NO4S | | |
| NH4HSO4 | haze in atmospheric chemistry |
| H5N3 | | |
| NH2NHNH2 | triazanes |
| H5P | | |
| PH5 | phosphoranes |
| H6S | | |
| SH6 | bonding number |
| H10N2Si3 | | |
| H3SiNHSiH2NHSiH3 | silazanes |
| He | | |
| He+ | Rutherford backscattering (RBS) |
| He | atomic laser |
| 4He | α-particle (alpha-particle) |
| 3He | helion |
| Hg | | |
| Hg | resonance lamp |
| I | | |
| I− | identity reaction |
| 131I | radioiodination |
| 125I | radioiodination |
| I+ | halonium ions |
| 129I | nuclear quadrupole moment (spectroscopic) |
| 123I | radioiodination |
| IK | | |
| IK | isotopic scrambling |
| IO2 | | |
| IO2 | characteristic group in organic nomenclature |
| K | | |
| K | syntectic reaction |
| KMnO4 | | |
| KMnO4 | equivalent entity |
| KZn | | |
| K-Zn | syntectic reaction |
| KZn13 | | |
| KZn13 | syntectic reaction |
| Kr | | |
| Kr | liquid ion laser |
| Kr | resonance lamp |
| La | | |
| La3+ | ionic conductivity |
| Li | | |
| Li+ | order-disorder transition |
| Li+ | order-disorder transition |
| LiMn2O4 | | |
| Li[Mn2]O4 | topochemical reaction |
| Mg | | |
| Mg2+ | selectivity coefficient,
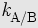 in ion exchange chromatography
in ion exchange chromatography |
| Mg | solute-volatilization interference
in flame spectroscopy |
| Mg2O4Si | | |
| Mg2SiO4 | reconstructive transition |
| Mg2SiO4 | reconstructive transition |
| MnO2 | | |
| MnO2 | magnetic transition |
| Mn3O4 | | |
| Mn3O4 | Jahn–Teller transition |
| N | | |
| N | alkoxyamines |
| ≡N | nitriles |
| N | umpolung |
| N | umpolung |
| N | dipolar compounds |
| N | dipolar compounds |
| N | dipolar compounds |
| N | dipolar compounds |
| N | imides |
| N | hydrogen bond |
| N | amidium ions |
| N | dipolar compounds |
| N | imides |
| NO | | |
| NO | reference procedure
in analysis of trace air constituents |
| NO+ | isoelectronic |
| NO | primary pollutant in atmospheric chemistry |
| NO | emission in atmospheric chemistry |
| –N=O | isonitroso compounds |
| NO | composition of pure air in atmospheric chemistry |
| –NO | nitroso compounds |
| NO2 | | |
| NO2+ | electrofuge |
| NO2 | composition of pure air in atmospheric chemistry |
| NO2 | permeation tube |
| NO2 | reference procedure
in analysis of trace air constituents |
| NO2 | π-electron acceptor/donor group |
| NO2 | air pollutant |
| NO2 | reference procedure
in analysis of trace air constituents |
| –NO2 | nitro compounds |
| NO2 | reference procedure
in analysis of trace air constituents |
| NO2 | electrophile (electrophilic) |
| NO3 | | |
| NO3− | mean activity of an electrolyte in solution |
| NO3 | oxidant in atmospheric chemistry |
| NO3Rb | | |
| RbNO3 | thermally-induced transition |
| N2 | | |
| N2 | isoelectronic |
| =N+=N− | diazo compounds |
| N2 | cage |
| N2 | reference procedure
in analysis of trace air constituents |
| N2 | geminate recombination |
| N2 | cryogenic sampling |
| N2 | molecular laser |
| N2O | | |
| N2O | local fraction atomized,
 , ,
 in flame emission and absorption spectrometry
in flame emission and absorption spectrometry |
| N3 | | |
| –N=N+=N− | azides |
| N3− | pseudohalogens |
| N3 | azides |
| –N3 | quinone diazides |
| N3Na | | |
| NaN3 | azides |
| Na | | |
| Na | resonance lamp |
| Na+ | mean activity of an electrolyte in solution |
| Na+ | interfering substance
in electroanalytical chemistry |
| Na+ | ionic conductivity |
| Nb3Sn | | |
| Nb3Sn | superconducting transition |
| Ne | | |
| Ne | helium–neon laser |
| Ne | atomic laser |
| Ni | | |
| 63Ni | electron capture detector in gas chromatography |
| NiS | | |
| NiS | dilational (dilatational) transition |
| O | | |
| –O– | imidines |
| =O | hydroximic acids |
| –O– | silathianes |
| O | umpolung |
| O | ylides |
| =O | imidines |
| O | umpolung |
| O | dipolar compounds |
| O | dipolar compounds |
| =O | oxo compounds |
| –O– | silazanes |
| O | dipolar compounds |
| =O | imidic acids |
| O | dipolar compounds |
| =O | hydrazidines |
| O | amidium ions |
| –O– | diamidides |
| O | imides |
| O | branching chain reaction |
| O | dipolar compounds |
| O | hydrogen bond |
| =O | hydrazones |
| O | tautomerism |
| O2− | ligands |
| O | imides |
| =O | diamidides |
| =O | characteristic group in organic nomenclature |
| OP | | |
| P–O | ligases (synthetases) |
| O2 | | |
| O2 | configuration (electronic) |
| O2 | biochemical (biological) oxygen demand ( ) ) |
| (=O)2 | sulfonediimines |
| O2 | flow rate
in flame emission and absorption spectrometry |
| O22− | peroxides |
| O2 | chemical oxygen demand ( ) ) |
| O2− | Haber–Weiss reaction |
| O2 | photosynthesis |
| O2 | catalase |
| O2 | flow rate
in flame emission and absorption spectrometry |
| O2 | singlet molecular oxygen |
| O2− | Haber–Weiss reaction |
| O2 | mean free path,

|
| O2 | singlet molecular oxygen |
| O2 | Haber–Weiss reaction |
| O2S | | |
| SO2 | oxidation state |
| SO2 | composition of pure air in atmospheric chemistry |
| SO2 | permeation tube |
| SO2 | air pollutant |
| SO2 | emission in atmospheric chemistry |
| SO2 | extraction (equilibrium) constant |
| SO2 | primary pollutant in atmospheric chemistry |
| O2S | cheletropic reaction |
| SO2 | oxidized species |
| SO2 | sink in atmospheric chemistry |
| O2Si | | |
| SiO2 | polymorphic transition |
| SiO2 | polymorphic transition |
| SiO2 | displacive transition |
| SiO2 | polymorphic transition |
| O2Ti | | |
| TiO2 | irreversible transition |
| TiO2 | irreversible transition |
| O2V | | |
| VO2 | metal–insulator transition |
| O2Zr | | |
| ZrO2 | martensitic transition |
| ZrO2 | martensitic transition |
| O3 | | |
| O3 | greenhouse effect in atmospheric chemistry |
| O3 | secondary pollution (emissions) |
| O3 | reference procedure
in analysis of trace air constituents |
| O3 | decay rate in atmospheric chemistry |
| O3S | | |
| SO3 | oxidation state |
| –(SO3−) | membrane sites
in an ion-selective electrode |
| SO3 | oxidized species |
| O3Ti2 | | |
| Ti2O3 | semiconductor-metal transition |
| O4S | | |
| SO42− | selectivity coefficient,
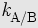 in ion exchange chromatography
in ion exchange chromatography |
| SO42− | Brønsted base |
| O4S3 | | |
| 3SO42− | mean activity of an electrolyte in solution |
| O4Si | | |
| SiO4 | displacive transition |
| O5P2 | | |
| P2O5 | electrolytic hygrometer |
| P | | |
| P | imides |
| P | umpolung |
| P | imides |
| PbS | | |
| PbS | photoconductive detector |
| Pt | | |
| Pt | gas sensing electrode |
| Pt | gas sensing electrode |
| S | | |
| S | mustards |
| S | triple point |
| S | ylides |
| S | polytypic transition |
| –S– | silathianes |
| S | umpolung |
| S | poison in catalysis |
| S | imides |
| S | tautomerism |
| S | imides |
| S | triple point |
| S | triple point |
| S | triple point |
| SZn | | |
| ZnS | polytypic transition |
| ZnS | polytypic transition |
| ZnS | polytypic transition |
| ZnS | polytypic transition |
| ZnS | polytypic transition |
| S2 | | |
| S2 | polysulfanes |
| S2Ti | | |
| TiS2 | intercalation reaction |
| S8 | | |
| S8 | oxidation state |
| Se | | |
| Se | ylides |
| Se | imides |
| Se | umpolung |
| Se | imides |
| Si | | |
| Si | silanols |
| Si | photoconductive detector |
| SiV3 | | |
| V3Si | superconducting transition |
| Sn | | |
| 119Sn | nuclear quadrupole moment (spectroscopic) |
| 119Sn | quadrupole splitting in Mössbauer spectroscopy |
| Te | | |
| Te | ylides |
| Te | imides |
| Te | imides |
| Ti | | |
| Ti3+ | solid state lasers |
| U | | |
| 239U | resonance neutrons |
| Xe | | |
| Xe | resonance lamp |
| Zn | | |
| Zn | resonance lamp |
| Zn | polytypic transition |
| Zn | syntectic reaction |

